��Ŀ����
��10�֣�������Ԫ�صĵ���X��Y��Z��ͨ��״���¾�Ϊ��̬��������ͼת����ϵ����Ӧ������ȥ������֪��
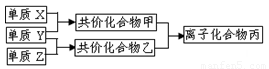
a������˫ԭ�ӵ��ʷ����У�X���Ӻ����ۼ���ࡣ
b�������к�10�����ӣ��ҷ��Ӻ���18�����ӡ�
��1��X�ĵ���ʽ��_______________________��
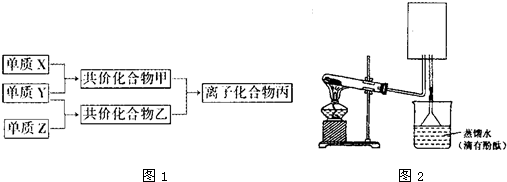
��2��ʵ���ҿ�����ͼ��ʾװ�ã�ȱ���ռ�װ�ã��г̶ֹ�װ����ȥ���Ʊ����ռ��ס�
����ͼ�з����ڻ���ռ�������װ�ü�ͼ��
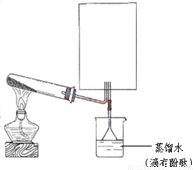
���Թ��е��Լ���____________________������д��ѧʽ��
���ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ��_________________________��
��3������Z��ȼ�տ��������ֲ������һ�ֲ��ﶡ�����и�ԭ������㲻ȫ��8���ӽṹ�����Ļ�ѧʽ��__________��
���𰸡�
��10�֣���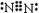 ���Ƣ�
���Ƣ� ����Ca��OH��2��NH4Cl����NH3��H2O
����Ca��OH��2��NH4Cl����NH3��H2O NH4++OH������PCl5
NH4++OH������PCl5
��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
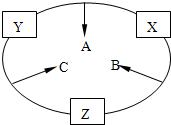 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯��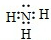


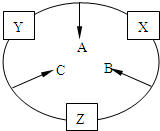 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��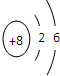
 NH4++OH-
NH4++OH-

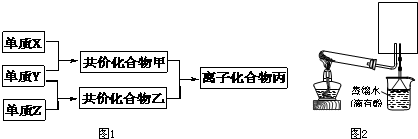 ������Ԫ�صĵ���X��Y��Z��ͨ��״���¾�Ϊ��̬����������ת����ϵ����Ӧ������ȥ����
������Ԫ�صĵ���X��Y��Z��ͨ��״���¾�Ϊ��̬����������ת����ϵ����Ӧ������ȥ����