��Ŀ����
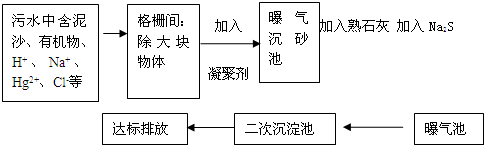
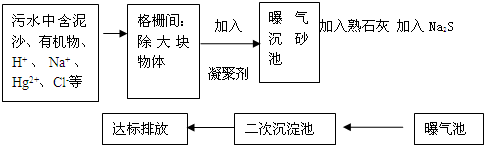
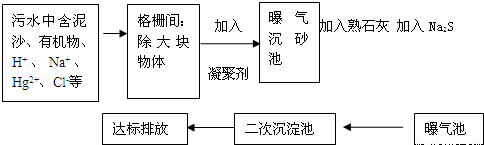
��ͼ��ij����ˮ�����Ĺ�������ʾ��ͼ��

��1�����õ����ۼ������� ������һ�����ۼ������ƻ�ѧʽ��
��2��������ʯ�ҵ�Ŀ���� ��д����صĻ�ѧ����ʽ ��
��3������Na2S�������� ��д����ص����ӷ���ʽ����дһ���� ��
��4��������ˮ�������ˮ����������ˮ���������������д��һ���������Ļ�ѧʽ ����������������������л������ʣ�������Ч���ã�
��5���ﵽ�ŷ�Ҫ���ˮ�к�����������Ҫ�� ��д��2�����ӷ��ţ���������������Ҫ�� ��д��2�����ӷ��ţ���

��1�����õ����ۼ�������
��2��������ʯ�ҵ�Ŀ����
��3������Na2S��������
��4��������ˮ�������ˮ����������ˮ���������������д��һ���������Ļ�ѧʽ
��5���ﵽ�ŷ�Ҫ���ˮ�к�����������Ҫ��
��������1���ھ�ˮʱʹ�õ���������������
��2����ʯ�ҵijɷ����������ƣ����к����Է�ˮ��
��3����ˮ����Hg2+���ӣ�����Na2S��ȥ��
��4��Ŀǰ����ˮ�����ö������Ȼ������������ɱ����
��5���ﵽ�ŷ�Ҫ���ˮ�к������������Ӷ��ǿ����Ե����ʵ�����ģ�
��2����ʯ�ҵijɷ����������ƣ����к����Է�ˮ��
��3����ˮ����Hg2+���ӣ�����Na2S��ȥ��
��4��Ŀǰ����ˮ�����ö������Ȼ������������ɱ����
��5���ﵽ�ŷ�Ҫ���ˮ�к������������Ӷ��ǿ����Ե����ʵ�����ģ�
����⣺��1����������ˮ�γɵĽ��������������������������������ã��Ǿ�ˮʱ���õ���������
�ʴ�Ϊ��������
��2����ʯ�ҵijɷ����������ƣ����к����Է�ˮ����ѧ����ʽΪCa��OH��2+2HCl=CaCl2+H2O��
�ʴ�Ϊ���к��ᣬCa��OH��2+2HCl=CaCl2+H2O��
��3����ˮ����Hg2+���ӣ�����Na2S��Ӧ������������ȥ����Ӧ����ʽΪ��Hg2++S2-=HgS����
�ʴ�Ϊ����ȥHg2+��Hg2++S2-=HgS��
��4��Ŀǰ����ˮ�����ö������Ȼ������������ɱ������������������������л������ʣ�������Ч���ã�
�ʴ�Ϊ��ClO2��O3��
��5���ﵽ�ŷ�Ҫ���ˮ�к�����������Ҫ��K+��Ca2+��Na+��������������Ҫ��Cl-��SO42-��
�ʴ�Ϊ��K+��Ca2+��Na+��Cl-��SO42-��
�ʴ�Ϊ��������
��2����ʯ�ҵijɷ����������ƣ����к����Է�ˮ����ѧ����ʽΪCa��OH��2+2HCl=CaCl2+H2O��
�ʴ�Ϊ���к��ᣬCa��OH��2+2HCl=CaCl2+H2O��
��3����ˮ����Hg2+���ӣ�����Na2S��Ӧ������������ȥ����Ӧ����ʽΪ��Hg2++S2-=HgS����
�ʴ�Ϊ����ȥHg2+��Hg2++S2-=HgS��
��4��Ŀǰ����ˮ�����ö������Ȼ������������ɱ������������������������л������ʣ�������Ч���ã�
�ʴ�Ϊ��ClO2��O3��
��5���ﵽ�ŷ�Ҫ���ˮ�к�����������Ҫ��K+��Ca2+��Na+��������������Ҫ��Cl-��SO42-��
�ʴ�Ϊ��K+��Ca2+��Na+��Cl-��SO42-��
���������⿼����ˮ�ľ�������ȷ���ʵ������ǽⱾ��ؼ���ע�������ܾ�ˮ������ɱ��������Ϊ�״��㣮

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ