��Ŀ����
��12�֣���֪N2��3H2 2NH3��������ͼ�жϣ�
2NH3��������ͼ�жϣ�
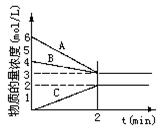
A��_____________�����ʵ���Ũ�ȱ仯�������Ӧ��ʼʱ��C(N2)="_____________" ��2minʱC(NH3)=_____________________��
0��2min��������ƽ����Ӧ����v(H2)= ___________________��
(2)һ�������£����淴ӦA2��B2 2C�ﵽ�˻�ѧƽ��״̬�������ƽ��ʱC��A2��= 0.5mol��L��C��B2��="0.1mol/L" C��C��=" 1.6" mol/L����A2��B2��C����ʼŨ�ȷֱ���a mol/L��b mol/L��cmol/L��ʾ����ش�
2C�ﵽ�˻�ѧƽ��״̬�������ƽ��ʱC��A2��= 0.5mol��L��C��B2��="0.1mol/L" C��C��=" 1.6" mol/L����A2��B2��C����ʼŨ�ȷֱ���a mol/L��b mol/L��cmol/L��ʾ����ش�
��a��bӦ����Ĺ�ϵ��______________��
��a��ȡֵ��Χ��________��˵������___________________________��
 2NH3��������ͼ�жϣ�
2NH3��������ͼ�жϣ�
A��_____________�����ʵ���Ũ�ȱ仯�������Ӧ��ʼʱ��C(N2)="_____________" ��2minʱC(NH3)=_____________________��
0��2min��������ƽ����Ӧ����v(H2)= ___________________��
(2)һ�������£����淴ӦA2��B2
 2C�ﵽ�˻�ѧƽ��״̬�������ƽ��ʱC��A2��= 0.5mol��L��C��B2��="0.1mol/L" C��C��=" 1.6" mol/L����A2��B2��C����ʼŨ�ȷֱ���a mol/L��b mol/L��cmol/L��ʾ����ش�
2C�ﵽ�˻�ѧƽ��״̬�������ƽ��ʱC��A2��= 0.5mol��L��C��B2��="0.1mol/L" C��C��=" 1.6" mol/L����A2��B2��C����ʼŨ�ȷֱ���a mol/L��b mol/L��cmol/L��ʾ����ش���a��bӦ����Ĺ�ϵ��______________��
��a��ȡֵ��Χ��________��˵������___________________________��
��ÿ��2�֣���12�֣�
��1�� H2 4mol/L 1.5mol/(L��min)��2��a- b=" 0.4" 0.4��a��1.3 ��
��1�� H2 4mol/L 1.5mol/(L��min)��2��a- b=" 0.4" 0.4��a��1.3 ��
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2Z(g)���¶ȷֱ�ΪT1��T2ʱ��X��ת���ʦ�(X) ��ʱ��仯�Ĺ�ϵ��ͼ�����н�����ȷ����
2Z(g)���¶ȷֱ�ΪT1��T2ʱ��X��ת���ʦ�(X) ��ʱ��仯�Ĺ�ϵ��ͼ�����н�����ȷ����
 5 kJ/mol
5 kJ/mol  ��������ţ�
��������ţ�
 2C��g������H<0��
2C��g������H<0�� xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��
xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2�� 2NH3��g��;��H=" " -92.2KJ��mol-1,��֪��ƽ��ʱ�����������ѹǿΪ��ʼ��80%.
2NH3��g��;��H=" " -92.2KJ��mol-1,��֪��ƽ��ʱ�����������ѹǿΪ��ʼ��80%. ��ƽ��״̬ʱ���ų�������Ϊ
��ƽ��״̬ʱ���ų�������Ϊ  __
__