��Ŀ����
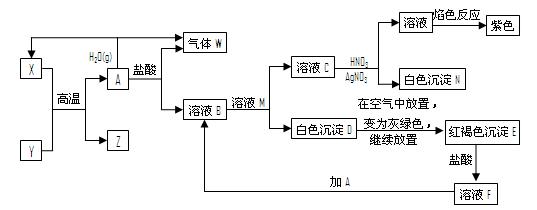
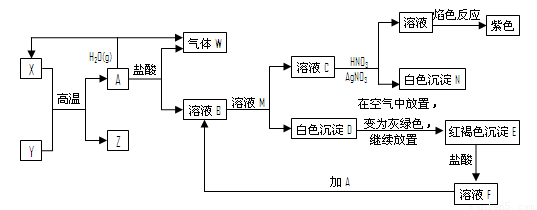
A��YΪ�����������ʣ�XΪ�����ǽ������ʡ�������X��G��H��IΪ���壬CΪҺ�塣B��������Ԫ����ɵ��Σ�����ʱ�����ֽ������������壬��ȴ���ֿɻ��ϵõ�B���й�����֮���ת����ϵ����ͼ�����ַ�Ӧ��������������ȥ��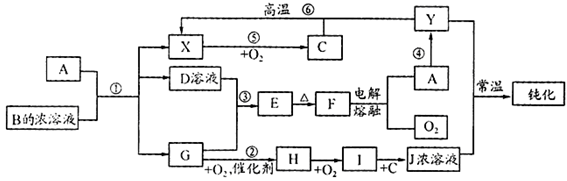
����д���пհף�
��1��B�ĵ���ʽΪ ��
��2��D��������ھ�ˮ��������Ӧ�����ӷ���ʽ��������˵��ԭ��
��
��3����Ӧ�Ļ�ѧ����ʽΪ ��
��Ӧ����ұ��ҵ������ ���������ұ����������
��4����D�Ľᾧˮ�����Ʊ�D����ˮ����IJ���Ϊ ��
��5����Ӧ�ڵĻ�ѧ����ʽΪ ��
��Ӧ�۵����ӷ���ʽΪ ��
��6���ռ�һ�Թ�H�����䵹����ˮ���У�Ȼ�����Թ���ͨ��һ������O2ʹ�Թ���Һ������������ʣ������ռ�Թ��ݻ���һ�룬��ԭ��H����ͨ��O2�������Ϊ ��
����6���⣬ ÿ��2�֣�
ÿ��2�֣�
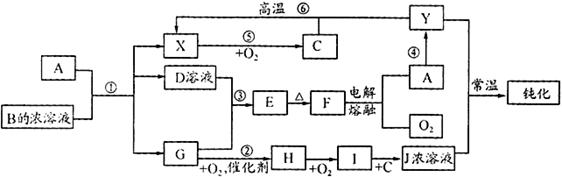
��1��NH4Cl�ĵ���ʽ��
��2��AlCl3��������ˮ�����������Al3+��ˮ��Al3++3H2O Al(OH)3(����)+3H+��
Al(OH)3(����)+3H+��
���ɵ�Al(OH)3���������ˮ�����ʣ��ﵽ��ˮĿ�ġ������ӷ���ʽ��д��ȷ��1�֣��ᵽAl(OH)3�����������1�֣�
��3��3Fe+4H2O(g) 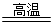 Fe3O4+4H2 �Ȼ�ԭ���������Ȼ�ԭ����
Fe3O4+4H2 �Ȼ�ԭ���������Ȼ�ԭ����
��4����D�Ľᾧˮ������HCl�����м��� ���ᵽHCl���弴�����֣�
��5��
Al3++3NH3��H2O��Al(OH)3��+3NH+ 4����Al3++3NH3+3H2O��Al(OH)3��+3NH+ 4��
��6��8��3��4��5 ����1�֣�
����

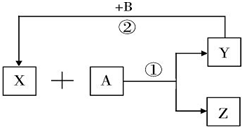 ���¸����ʾ�Ϊ��ѧ�γ��������ʣ�����B�ǵ��ʣ����ǵ�ת����ϵ��ͼ��ʾ��
���¸����ʾ�Ϊ��ѧ�γ��������ʣ�����B�ǵ��ʣ����ǵ�ת����ϵ��ͼ��ʾ��