��Ŀ����
��һ��������þ��ͭ�Ͻ���뵽ϡ������Һ�У�����ǡ����ȫ��Ӧ�����跴Ӧ�����л�ԭ����ȫ����NO����������Һ�м������ʵ���Ũ��Ϊ3mol?L-1��NaOH��Һ��������ȫ��������ɳ�����������ԭ�Ͻ����������5.1g���������й���������ȷ���ǣ�������
| A�����ɳ�����ȫʱ����NaOH��Һ�����Ϊ100mL |
| B���μӷ�Ӧ����������ʵ���Ϊ0.1mol |
| C����ʼ����Ͻ����������Ϊ16.4g |
| D������²���NO�����Ϊ22.4L |
��һ������þ��ͭ�Ͻ���뵽ϡHNO3�У�����ǡ�÷�Ӧ�����������ᶼû��ʣ�࣬��Ӧ�л�ԭ����ֻ��NO��������Ӧ��3Mg+8HNO3��ϡ���T3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3��ϡ���T3Cu��NO3��2+2NO��+4H2O��
��Ӧ�����Һ�м���3mol/LNaOH��Һ��������ȫ��������Ӧ��Mg��NO3��2+2NaOH�TMg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH�TCu��OH��2��+2NaNO3��
����Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������5.1g��
��������þ��������ͭ����������������Ϊ5.1g�������������ʵ���Ϊ
=0.3mol��
���ݵ���ת���غ㣬��þ��ͭ���ܵ����ʵ���Ϊ
=0.15mol������NOΪ
=0.1mol��
A���������������ǡ��������þ������ͭ��Ӧ��������������֪�������NaOHΪ0.3mol���ʼ���NaO��Һ�����Ϊ
=0.1L=100mL����A��ȷ��
B�����ݷ���ʽ��֪�μӷ�Ӧ��n��Ӧ��HNO3��=
n��������=
��0.15mol=0.4mol����B����
C��þ��ͭ���ܵ����ʵ���Ϊ0.15mol���ٶ�ȫΪþ������Ϊ0.15mol��24g/mol=3.6g����ȫΪͭ������Ϊ0.15mol��64g/mol=9.6g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ3.6g��m��9.6g����C����
D�������NO�����Ϊ0.1mol��22.4L/mol=2.24L����D����
��ѡ��A��
��Ӧ�����Һ�м���3mol/LNaOH��Һ��������ȫ��������Ӧ��Mg��NO3��2+2NaOH�TMg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH�TCu��OH��2��+2NaNO3��
����Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������5.1g��
��������þ��������ͭ����������������Ϊ5.1g�������������ʵ���Ϊ
| 5.1g |
| 17g/mol |
���ݵ���ת���غ㣬��þ��ͭ���ܵ����ʵ���Ϊ
| 0.3mol |
| 2 |
| 0.3mol |
| 5-2 |
A���������������ǡ��������þ������ͭ��Ӧ��������������֪�������NaOHΪ0.3mol���ʼ���NaO��Һ�����Ϊ
| 0.3mol |
| 3mol/L |
B�����ݷ���ʽ��֪�μӷ�Ӧ��n��Ӧ��HNO3��=
| 8 |
| 3 |
| 8 |
| 3 |
C��þ��ͭ���ܵ����ʵ���Ϊ0.15mol���ٶ�ȫΪþ������Ϊ0.15mol��24g/mol=3.6g����ȫΪͭ������Ϊ0.15mol��64g/mol=9.6g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ3.6g��m��9.6g����C����
D�������NO�����Ϊ0.1mol��22.4L/mol=2.24L����D����
��ѡ��A��

��ϰ��ϵ�д�
�����Ŀ
 ��
�� ��
��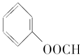 ��CH3OH����HCOOH��
��CH3OH����HCOOH��