��Ŀ����
��10�֣�ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
����1����Ҫ��ȡŨ���� ������ mL(ȷ��С�����һλ)��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ���200mL����ƿ�⣬�������õ��������� ������ �� ������ �ȡ�
��3������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ������( )
A�������˽���Ľ�������ʹ������ƿ
B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ��
C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶��߸Ŀ�������ƿ��ƿ����
D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ��
��4�������ƹ����У��������ض������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿
������ƿδ���T����������Һ����������ҺŨ�� ���� 0.9 mol��L-1������ڡ��������ڡ���С�ڡ�����ͬ����������ʱ���ӿ̶��ߣ���������ҺŨ�� ���� 0.9 mol��L-1��
����10�֣��� ��1��10.0 (2��)
��2�������� ��ͷ�ιܣ��ɵߵ�����(ÿ��1��)
��3��B��2�֣� ��4������ ���ڣ�ÿ��2�֣�
��������
�����������1����Ũ�����ϡ�����У������Dz���ģ�������Ҫ��ȡŨ����������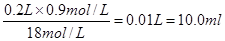 ��
��
��2��ϡ��Ũ���ᡢ������ƿ��ת������ʱ����Ҫ�����������ⶨ��ʱ����Ҫ��ͷ�ιܡ�
��3����������ƿ�Ľṹ��֪��ƿ��Խ�֣���ȷ��Խ�ͣ����Ըý��鲻��ȡ����ѡB��
��4������n��c��V��֪���������ƿû�и�������ʵ���Һ���������Ӱ�죬Ũ�Ȳ��䣻�������ʱ���ӿ̶��ߣ���������Һ�����ƫ�٣�Ũ��ƫ�ߡ�
���㣺����һ�����ʵ���Ũ����Һ�����ơ�������ѡ�����ʵ���Ũ�ȵļ����������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣������ؿ���ѧ���Ļ���֪ʶ����������Ŀ��顣������Ѷ�����������������һ�����ʵ���Ũ����Һ��ʵ������ѧ��ѧ��һ����Ҫ�Ķ���ʵ�飬ʵ�������������ҺŨ�ȴ������������кܶࡣ�Ӵ�ķ��潲��һ����ʵ������еIJ��淶��������ģ�������������ҩƷ��ϵͳԭ������ġ�������������ԭ���ӣ������������ͳ�Ϊ�߿���ѧʵ���е�һ���ѵ㡣

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д���10�֣�ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
����1����Ҫ��ȡŨ���� ������ mL(ȷ��С�����һλ)��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ���200mL����ƿ�⣬�������õ��������� ������ �� ������ �ȡ�
��3������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ������( )
| A�������˽���Ľ�������ʹ������ƿ |
| B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ�� |
| C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶��߸Ŀ�������ƿ��ƿ���� |
| D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ�� |
������ƿδ���T����������Һ����������ҺŨ�� ���� 0.9 mol��L-1������ڡ��������ڡ���С�ڡ�����ͬ����������ʱ���ӿ̶��ߣ���������ҺŨ�� ���� 0.9 mol��L-1��