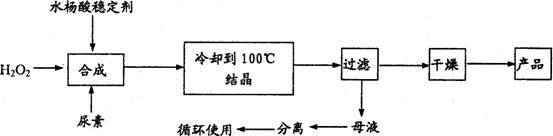
��Ŀ����
����������[CO��NH2��2?H2O2]��һ��������ζ�İ�ɫ�ᾧ��ĩ���������غ�������˫�����ʣ���һ�����͵������������������㷺Ӧ����Ư�ס���֯��ҽҩ��ũҵ����ֳҵ��������ϳ����£�
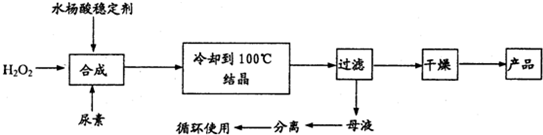
�Իش��������⣺
��1��ʵ�������������n��H2O2����n��CO��NH2��2��]=1.2��1�������ƺϳ��¶���25-30�棬����Ҫԭ����______��
��2����ĸҺ�з����H2O2�����أ����õIJ�����______��
��a������ ���� ��b����Һ ���� ��c����ѹ���� �ᾧ ��d����ѹ���� ��ȡ
��3��Ϊ�ⶨ��Ʒ�л������ĺ�����������16%���൱��H2O234%������ȡ������Ʒ12.000g���ܽ⣬��250mL����ƿ�ж��ݣ�ȷ��ȡ25.00mL����ƿ�У�����1mL 6mol/L�����ᣬȻ����0.2000mol/L KMnO4 ����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ20.00mL��KMnO4��Һ�����ز���Ӧ����
��KMnO4��ҺӦʢ����______ʽ��ѡ����ᡱ��������ζ����У�
����ɲ���ƽ����ʽ��______MnO4-+______H2O2+______H+=______Mn2++______H2O+______
�۸��ݵζ��������ȷ����Ʒ�л���������������Ϊ��______��
�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õĻ���������______��ѡ���ƫ�ߡ�����ƫ�͡����䡱����
�ݸ��ݱ���ʵ���õĻ��������������жϸ�ʵ���Ʒ������һ����Ҫ����Ϊ______��
�⣺��1����һ���¶��·�����Ӧʱ���ȹ�������ֽ⣬������������������߷�Ӧת���ʣ���߹��������صĴ��ȣ�
�ʴ�Ϊ��H2O2 ��ʵ������л��в��ַֽ⣬������������������߹��������صĴ��ȣ�
��2��Һ��ķе���ָ��������ѹ�������ѹ��ʱ���¶ȣ����Һ��ķе��������ѹ���ı仯���仯�ģ������������ձý���ϵͳ��ѹ�����Ϳ��Խ���Һ��ķе㣮H2O2��ѹ�·е�108�ȣ����س�ѹ��169.6�ȣ���е�͵��ȷ��ڣ�����Һ̬��Ϊ��̬����ΪH2O2�����ֽ⣬���Դ�ĸҺ�з����H2O2������ʱ��Ӧʹ��Һ�ڽϵ��¶������������õIJ����Ǽ�ѹ����Ȼ��ᾧ���ʴ�Ϊ��c��
��3����KMnO4��Һ����ǿ�����ԣ��ܽ���ʽ�ζ����¶˵������������Բ����ü�ʽ�ζ�����ȡ����������ʽ�ζ�����ȡ���ʴ�Ϊ���
�ڷ�Ӧ��MnO4-����������H2O2�ǻ�ԭ��������������O2������Ԫ�ػ��ϼ۱仯����Ԫ�ػ��ϼ۴�+7�۱仯Ϊ+2�ۣ����������е���Ԫ�ػ��ϼ۴�-1�۱仯Ϊ0�ۣ����ݵ����غ���ƽд�����ӷ���ʽΪ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2���ʴ�Ϊ��2��5��6��2��8��5O2��
�۳�ȡ������Ʒ12.000g���ܽ⣬��250mL����ƿ�ж��ݣ�ȷ��ȡ25.00mL����ƿ�У�����1mL 6mol/L�����ᣬȻ����0.2000mol/L KMnO4 ����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ20.00mL�����ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2MnO4-����������5H2O2��
2 5
0.0200L��0.2000mol/L 0.01mol
250mL����ƿ�к���������0.1mol��
����������������= ��100%=28.3%
��100%=28.3%
������16%���൱��H2O234%�����������������= =13.3%��
=13.3%��
�ʴ�Ϊ��13.3%��
�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ�����ݷ�Ӧ�ĵ����ϵ��֪��5��c����V��=c�����V�����2����ñ�Һ���ƫ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
���������̷�����ĸҺ���ܷ����H2O2�����أ��������ԭ�ϣ�ѭ��ʹ�ã�����ȴ�ᾧ�����л��������ھ��������������Ը�ʵ���Ʒ������һ����Ҫ���������أ�
�ʴ�Ϊ�����ػ�ˮ�����ȶ�����
��������1�����ݷ�Ӧ��һ���¶��·�����Ӧʱ���ȹ�������ֽ������CO��NH2��2+H2O2=CO��NH2��2?H2O2��
��2���������ʷе㲻ͬ��Һ��ķе��������ѹ���ı仯���仯�ķ����жϣ�
��3��������KMnO4��Һ��ǿ�����Ժ����ԣ�Ӧʢ������ʽ�ζ����У�
������������ԭ��Ӧ�����غ�͵���غ㣬�����غ㣬ԭ���غ���ƽд�����ӷ���ʽ��
������2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����������������ʵ�������������Ϣ�����Ʒ�л�����������������
�����ݵζ��������������ݣ�c����V��=c�����V���⣬��������ԭ���DZ�Һ���ĵĶ��ٷ����жϣ�
����������ͼ�кͷ������ʵķ��������жϣ�
���������⿼���������Ʊ������̷����жϣ��ζ��ܵ�ʹ�÷����ͽṹ�������ζ�ʵ��ļ���Ӧ�ã����̷����ǽ���ؼ�����Ŀ�Ѷ��еȣ�
�ʴ�Ϊ��H2O2 ��ʵ������л��в��ַֽ⣬������������������߹��������صĴ��ȣ�
��2��Һ��ķе���ָ��������ѹ�������ѹ��ʱ���¶ȣ����Һ��ķе��������ѹ���ı仯���仯�ģ������������ձý���ϵͳ��ѹ�����Ϳ��Խ���Һ��ķе㣮H2O2��ѹ�·е�108�ȣ����س�ѹ��169.6�ȣ���е�͵��ȷ��ڣ�����Һ̬��Ϊ��̬����ΪH2O2�����ֽ⣬���Դ�ĸҺ�з����H2O2������ʱ��Ӧʹ��Һ�ڽϵ��¶������������õIJ����Ǽ�ѹ����Ȼ��ᾧ���ʴ�Ϊ��c��
��3����KMnO4��Һ����ǿ�����ԣ��ܽ���ʽ�ζ����¶˵������������Բ����ü�ʽ�ζ�����ȡ����������ʽ�ζ�����ȡ���ʴ�Ϊ���
�ڷ�Ӧ��MnO4-����������H2O2�ǻ�ԭ��������������O2������Ԫ�ػ��ϼ۱仯����Ԫ�ػ��ϼ۴�+7�۱仯Ϊ+2�ۣ����������е���Ԫ�ػ��ϼ۴�-1�۱仯Ϊ0�ۣ����ݵ����غ���ƽд�����ӷ���ʽΪ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2���ʴ�Ϊ��2��5��6��2��8��5O2��
�۳�ȡ������Ʒ12.000g���ܽ⣬��250mL����ƿ�ж��ݣ�ȷ��ȡ25.00mL����ƿ�У�����1mL 6mol/L�����ᣬȻ����0.2000mol/L KMnO4 ����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ20.00mL�����ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2MnO4-����������5H2O2��
2 5
0.0200L��0.2000mol/L 0.01mol
250mL����ƿ�к���������0.1mol��
����������������=
 ��100%=28.3%
��100%=28.3%������16%���൱��H2O234%�����������������=
 =13.3%��
=13.3%���ʴ�Ϊ��13.3%��
�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ�����ݷ�Ӧ�ĵ����ϵ��֪��5��c����V��=c�����V�����2����ñ�Һ���ƫ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
���������̷�����ĸҺ���ܷ����H2O2�����أ��������ԭ�ϣ�ѭ��ʹ�ã�����ȴ�ᾧ�����л��������ھ��������������Ը�ʵ���Ʒ������һ����Ҫ���������أ�
�ʴ�Ϊ�����ػ�ˮ�����ȶ�����
��������1�����ݷ�Ӧ��һ���¶��·�����Ӧʱ���ȹ�������ֽ������CO��NH2��2+H2O2=CO��NH2��2?H2O2��
��2���������ʷе㲻ͬ��Һ��ķе��������ѹ���ı仯���仯�ķ����жϣ�
��3��������KMnO4��Һ��ǿ�����Ժ����ԣ�Ӧʢ������ʽ�ζ����У�
������������ԭ��Ӧ�����غ�͵���غ㣬�����غ㣬ԭ���غ���ƽд�����ӷ���ʽ��
������2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����������������ʵ�������������Ϣ�����Ʒ�л�����������������
�����ݵζ��������������ݣ�c����V��=c�����V���⣬��������ԭ���DZ�Һ���ĵĶ��ٷ����жϣ�
����������ͼ�кͷ������ʵķ��������жϣ�
���������⿼���������Ʊ������̷����жϣ��ζ��ܵ�ʹ�÷����ͽṹ�������ζ�ʵ��ļ���Ӧ�ã����̷����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ