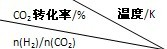
��Ŀ����
I����80��ʱ����0.4mol�������������������2L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�| ʱ��/s c/mol?L-1 | 20 | 40 | 60 | 80 | 100 | |
| N2O4 | 0.20 | a | 0.10 | c | d | e |
| NO2 | 0.12 | b | 0.26 | 0.30 | 0.30 |
��1������b______c�����������=��������������0��20s��N2O4��ƽ����Ӧ����Ϊ______��
��2��80��ʱ���÷�Ӧ��ƽ�ⳣ��K=______��
��3��������������ͬʱ���÷�Ӧ��KֵԽ��������ƽ��ʱ______��
A��N2O4��ת����Խ��B��NO2�IJ���Խ��
C��N2O4��NO2��Ũ��֮��Խ��D������Ӧ���еij̶�Խ��
��4��Ҫ����÷�Ӧ��Kֵ���ɲ�ȡ�Ĵ�ʩ______��
A������N2O4��ʼŨ�� B����������ͨ��NO2
C��ʹ�ø�Ч����D�������¶�
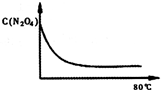
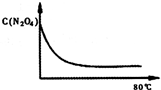
��5����ͼ��80��ʱ������N2O4Ũ�ȵı仯ͼ�����ڸ�ͼ�в������÷�Ӧ��60�淴ӦʱN2O4Ũ�ȵı仯���ߣ�
��2�����ݻ�ѧ��Ӧ��ƽ�ⳣ���Ĺ�ʽ�����
��3����4�����ݻ�ѧƽ�ⳣ��Kֵ�뻯ѧƽ���ƶ��Ĺ�ϵ��
��5�������¶ȶԻ�ѧ��Ӧ���ʺͻ�ѧƽ���Ӱ��
����⣺��1��N2O4
 2NO2
2NO2��ʼ��mol?L-1 �� 0.20 0
��Ӧ��mol?L-1 �� 0.10 0.20
ĩ�ˣ�mol?L-1 �� 0.10 b
b=0.20
N2O4
 2NO2
2NO2��ʼ��mol?L-1 �� 0.20 0
��Ӧ��mol?L-1 �� 0.13 0.26
ĩ�ˣ�mol?L-1 �� c 0.26
c=0.07
��� b��c
����0��20s��NO2��ƽ����Ӧ����Ϊv��NO2��=
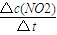 =
= =0.006mol?��L?s��-1
=0.006mol?��L?s��-1��0��20s0��20s��N2O4ƽ����Ӧ����Ϊv��N2O4��=
 v��N2O4��=0.003mol?��L?s��-1
v��N2O4��=0.003mol?��L?s��-1�ʴ�Ϊ������0.003mol?��L?s��-1��
��2��N2O4
 2NO2
2NO2��ʼ��mol?L-1 �� 0.20 0
��Ӧ��mol?L-1 �� 0.15 0.30
ĩ�ˣ�mol?L-1 �� 0.05 0.30
�÷�Ӧ��ƽ�ⳣ��K=
 =
= =1.8
=1.8�ʴ�Ϊ��1.8
��3������ƽ�ⳣ��K����˵����ѧƽ�������ƶ�������Ӧ���еij̶�����N2O4��ת��������NO2�IJ�������N2O4��NO2��Ũ��֮�ȼ�С����ѡ��A��B��D
��4��Ҫ����÷�Ӧ��Kֵ������ʹ��ѧƽ�������ƶ������¶Ȳ���ʱ���ı�Ũ�ȡ�ѹǿ����ʹƽ�ⷢ���ƶ�����ƽ�ⳣ���������ı䣬ʹ�ø�Ч������ƽ�ⲻ�����ƶ���ƽ�ⳣ�����䣬ֻ�иı��¶ȣ�ƽ�ⷢ���ƶ�ʱ��Kֵ�ŷ����ı䣬��ѡ��D
��5���¶Ƚ��ͣ���ѧ��Ӧ���ʼ���������ƽ���ʱ�䳤��ͬʱ���¶Ƚ��ͣ���ѧƽ������ȵķ����ƶ������淴Ӧ�����ƶ�������ͼ

������������Ҫ�����˻�ѧ��Ӧ���ʣ�ƽ�ⳣ���뻯ѧƽ���ƶ��Ĺ�ϵ��������ۺ��Խ�ǿ����һ�����Ѷȣ�

I.��ѧ��һֱ�����ڡ��˹��̵����ķ����о���Ŀǰ�ϳɰ��ļ���ԭ��Ϊ�����������ڸ��¸�ѹ�������������ɰ�����һ�������£���һ��2L���ܱ������г���2molN2��6molH2����Ӧ��ƽ��ʱ����NH3��Ũ��Ϊ0.5mol/L�����ų�QkJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ɱ�ʾΪ______��
II.��֪��N2O4(g)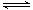 2NO2(g)
��H����57.20kJ/mol��
2NO2(g)
��H����57.20kJ/mol��
��1000Cʱ����0.100molN2O4�������lL���ݳ�յ��ܱ������У�ÿ��һ��ʱ��Ը������ڵ�����Ũ�Ƚ��з����õ��±����ݣ�
|
ʱ�䣨s�� |
0 |
20 |
40 |
60 |
80 |
|
c(N2O4)/mol |
0.100 |
c1 |
0.050 |
c3 |
c4 |
|
c(NO2)/mol |
0.000 |
0.060 |
c2 |
0.120 |
0.120 |
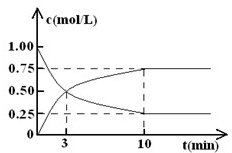
(1)�÷�Ӧ��ƽ�ⳣ������ʽΪ______���ӱ������ݷ�����c1 ______c2��c3______c4(ѡ�>������<����=������
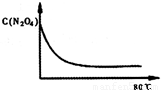
(2)����ͼ�л����������˷�Ӧ��N2O4��NO2��Ũ����ʱ��仯�����ߡ�

(3)�����������£��ӷ�Ӧ��ʼ���ﵽ��ѧƽ��ʱ��������������ƽ����Ӧ����Ϊ______��
(4)����ʼʱ����NO2����0.200mol����ﵽƽ��ʱNO2 �����ת����Ϊ______��������������ʱ�����д�ʩ�����NO2ת���ʵ���______ (����ĸ����
A.��СNO2��Ũ�� B.�����¶� C.����NO2��Ũ��
D.�����¶� E.�ٳ���һ������He
(5)���ݻ���ͬ���¶ȷֱ�ΪT1��T1�������ܱ������зֱ�������NO2��������Ӧ��2NO2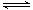 N2O4(g) (g) ��H����57.20kJ/mol�����º����·�Ӧ��ͬʱ��ֱ�����ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1<T2,��a1____a2
(ѡ��A��B��C��D��գ���
N2O4(g) (g) ��H����57.20kJ/mol�����º����·�Ӧ��ͬʱ��ֱ�����ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1<T2,��a1____a2
(ѡ��A��B��C��D��գ���
A.���� B.С�� C.���� D.���϶��п���
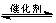 CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011
CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011