��Ŀ����
��2013?տ����ģ������ʯ����ѧʽ��ʾΪMgCO3?CaCO3��Ϊԭ���Ʊ�Mg��OH��2�Ĺ�����������ͼ��ʾ��

��1����ĥ��������
��2���ù����п�ѭ��ʹ�õ�������
��3������ʯ���յ���Ҫ������MgO?CaCO3������ͳ�����ǽ�����ʯ���ȷֽ�ΪMgO��CaO����ȡ������ʯ���յ��ŵ���
��4�����ȷ�Ӧ�����ӷ���ʽΪ
��5���ټ��ȷ�Ӧʱ����323k��353k��Һ��c��NH4+���뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ��������ͼ����373k�����ߣ�

����ͼ��֪�������¶����ߣ�

��1����ĥ��������
�������ı�������ӿ췴Ӧ���ʣ�����ԭ��������
�������ı�������ӿ췴Ӧ���ʣ�����ԭ��������
����2���ù����п�ѭ��ʹ�õ�������
NH3
NH3
����NH4��2SO4
��NH4��2SO4
��д��ѧʽ������3������ʯ���յ���Ҫ������MgO?CaCO3������ͳ�����ǽ�����ʯ���ȷֽ�ΪMgO��CaO����ȡ������ʯ���յ��ŵ���
�����ܺġ�����CO2���ŷŵȣ������ܡ���̼��
�����ܺġ�����CO2���ŷŵȣ������ܡ���̼��
����4�����ȷ�Ӧ�����ӷ���ʽΪ
MgO+2NH4+=Mg2++2NH3��+H2O
MgO+2NH4+=Mg2++2NH3��+H2O
����5���ټ��ȷ�Ӧʱ����323k��353k��Һ��c��NH4+���뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ��������ͼ����373k�����ߣ�

����ͼ��֪�������¶����ߣ�
��Ӧ��ʱ�����̡�ƽ��ʱc��NH4+����С
��Ӧ��ʱ�����̡�ƽ��ʱc��NH4+����С
����������1������Ӱ�컯ѧ��Ӧ���ʵ����ط�������ĥ����Ӵ��������Ӧ���ʣ�
��2����������ͼ���������ڷ�Ӧ�����������ɵ����ʿ���ѭ�����ã�
��3�����ݽ��ܼ��ŷ��������� ���յ�Ŀ�ģ�
��4�����յõ�MgO?CaCO3����������狀�ˮ��Ӧ���ɰ���������笠�����ˮ���������ܽ�����þ��
��5�����¶����߰���Ũ�ȼ�С���ﵽƽ������Ҫ��ʱ�����̣��ݴ˻������ߣ�
��ͼ������¶����������������̴ﵽƽ���ʱ�䣬笠�����Ũ�ȼ�С��
��2����������ͼ���������ڷ�Ӧ�����������ɵ����ʿ���ѭ�����ã�
��3�����ݽ��ܼ��ŷ��������� ���յ�Ŀ�ģ�
��4�����յõ�MgO?CaCO3����������狀�ˮ��Ӧ���ɰ���������笠�����ˮ���������ܽ�����þ��
��5�����¶����߰���Ũ�ȼ�С���ﵽƽ������Ҫ��ʱ�����̣��ݴ˻������ߣ�
��ͼ������¶����������������̴ﵽƽ���ʱ�䣬笠�����Ũ�ȼ�С��
����⣺��1����ĥ�������ʵĽӴ��������Ӧ������������ԭ�ϵ������ʣ��ʴ�Ϊ���������ı�������ӿ췴Ӧ���ʣ�����ԭ�������ʣ�
��2���������̷�����֪�������Ȼ���ڷ�Ӧ�����вμӷ�Ӧ����Ӧ�����������ɣ�����ѭ�����ã��ʴ�Ϊ��NH3����NH4��2SO4��
��3�����ռ����ܺĺͶ�����̼������ŷţ����Ͻ��ܼ��ŵ�Ŀ�ģ��ʴ�Ϊ�������ܺġ�����CO2���ŷŵȣ�
��4�����յõ�MgO?CaCO3����������狀�ˮ��Ӧ���ɰ���������笠�����ˮ���������ܽ�����þ��Ӧ�����ַ���ʽΪ��MgO+2NH4+=Mg2++2NH3��+H2O��
�ʴ�Ϊ��MgO+2NH4+=Mg2++2NH3��+H2O��
��5�����¶����߰���Ũ�ȼ�С���ﵽƽ������Ҫ��ʱ�����̣��ݴ˻������ߣ�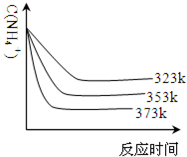 ��
��
�ʴ�Ϊ�� ��
��
��ͼ�������֪�¶�Խ�ߣ���Ӧ�������ﵽƽ������Ҫ��ʱ�����̣�笠� ���ֵ�Ũ�ȼ��٣��ʴ�Ϊ����Ӧ��ʱ�����̡�ƽ��ʱc��NH4+����С��
��2���������̷�����֪�������Ȼ���ڷ�Ӧ�����вμӷ�Ӧ����Ӧ�����������ɣ�����ѭ�����ã��ʴ�Ϊ��NH3����NH4��2SO4��
��3�����ռ����ܺĺͶ�����̼������ŷţ����Ͻ��ܼ��ŵ�Ŀ�ģ��ʴ�Ϊ�������ܺġ�����CO2���ŷŵȣ�
��4�����յõ�MgO?CaCO3����������狀�ˮ��Ӧ���ɰ���������笠�����ˮ���������ܽ�����þ��Ӧ�����ַ���ʽΪ��MgO+2NH4+=Mg2++2NH3��+H2O��
�ʴ�Ϊ��MgO+2NH4+=Mg2++2NH3��+H2O��
��5�����¶����߰���Ũ�ȼ�С���ﵽƽ������Ҫ��ʱ�����̣��ݴ˻������ߣ�
 ��
���ʴ�Ϊ��
 ��
����ͼ�������֪�¶�Խ�ߣ���Ӧ�������ﵽƽ������Ҫ��ʱ�����̣�笠� ���ֵ�Ũ�ȼ��٣��ʴ�Ϊ����Ӧ��ʱ�����̡�ƽ��ʱc��NH4+����С��
���������⿼���������Ʊ����������ʵķ���Ӧ�ã��Ʊ����ʵ����̹�ϵ�ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
�����Ŀ