��Ŀ����
I��������������ȷ����A��CS2ΪV�εļ��Է��� B��Cl0-3 �Ŀռ乹��Ϊƽ��������
C��SF6����6����ȫ��ͬ�ijɼ����Ӷ� D��SiF4��SO2-3 ������ԭ�Ӿ�Ϊsp3�ӻ�
������˵���д�����ǣ�
A��SO2��SO3���Ǽ��Է���
B����NH4+ ��[Cu��NH3��4]2+�ж�������λ��
C��Ԫ�ص縺��Խ���ԭ�ӣ��������ӵ�����Խǿ
D��ԭ�Ӿ�����ԭ���Թ��ۼ���ϣ����м��ܴ��۵�ߡ�Ӳ�ȴ������
����������Raney-Ni����һ����ʷ�ƾá�Ӧ�ù㷺�Ĵ���������-���Ͻ�Ϊԭ���Ƶã�
��1��Ԫ�ص�һ�����ܣ�Al
��2������������һʵ��Ϊ��
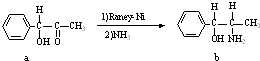
������b�н���sp3�ӻ���ԭ���У�
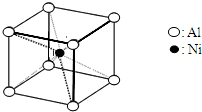
��3��һ�������Ͻ�Ľṹ��ͼ������ṹ���ƵĻ������ǣ�
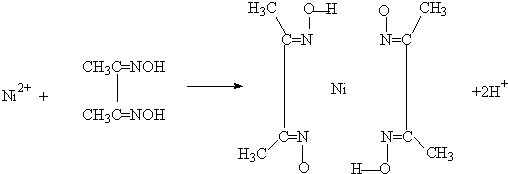
��4��ʵ���Ҽ���Ni2+���ö���ͪ���֮���������Ⱥ�ɫ����������
��Ni2+�ڻ�̬ʱ����������Ų�ʽΪ��
������������û�ѧ����������δ��������������������λ��Ϊ4����
�����������ݼ۲���ӶԻ������ۿ�֪CS2Ϊֱ���εķǼ��Է��ӣ�Cl03-�������Σ���ԭ���������6�����ӣ��ͷ�ԭ��֮����6����ȫ��ͬ�ijɼ����Ӷԣ�SiF4��SO32- �Ŀռ乹�ͷֱ�Ϊ��������������Σ�������ԭ�Ӿ����õ���sp3�ӻ���
������λ�����縺�ԡ����Է��ӵĸ����Լ�ԭ�Ӿ�����۷е�֪ʶ�����
��1��MgԪ��ԭ�ӵ�3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬���������ͣ�
��2�������д��ڴ�м���̼ԭ���ӻ���Ϊ2����ȡsp2�ӻ���������C��O��Nԭ�Ӹ��ݳɵĵ�����Ŀ��¶Ե���ȷ���ӻ������Ŀ�ж��ӻ���ʽ��
��3����ͼ��֪�����Ͻ�ľ����ṹ�У�Niԭ����ĿΪ1��Alԭ����ĿΪ8��
=1��ΪAlNi��ÿ��Niԭ����Χ��8��Alԭ�ӣ�ÿ��Alԭ����Χ��8��Niԭ�ӣ��ݴ˽��ѡ���и����ʵľ����ṹ�жϣ�
��4���ٸ��ݺ�������Ų�������д��
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ԭ������ԭ��֮���γ������
������λ�����縺�ԡ����Է��ӵĸ����Լ�ԭ�Ӿ�����۷е�֪ʶ�����
��1��MgԪ��ԭ�ӵ�3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬���������ͣ�
��2�������д��ڴ�м���̼ԭ���ӻ���Ϊ2����ȡsp2�ӻ���������C��O��Nԭ�Ӹ��ݳɵĵ�����Ŀ��¶Ե���ȷ���ӻ������Ŀ�ж��ӻ���ʽ��
��3����ͼ��֪�����Ͻ�ľ����ṹ�У�Niԭ����ĿΪ1��Alԭ����ĿΪ8��
| 1 |
| 8 |
��4���ٸ��ݺ�������Ų�������д��
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ԭ������ԭ��֮���γ������
����⣺��A�����ݼ۲���ӶԻ������ۿ�֪CS2Ϊֱ���εķǼ��Է��ӣ���A����
B���ɼ۲���ӶԻ������ۿ�֪Cl03-������ԭ�ӵŵ��Ӷ�����
����8-3��2��=1������Cl03-����������B����
C����ԭ���������6�����ӣ��ͷ�ԭ��֮����6����ȫ��ͬ�ijɼ����Ӷԣ���C��ȷ��
D��SiF4��SO32- �Ŀռ乹�ͷֱ�Ϊ��������������Σ�������ԭ�Ӿ����õ���sp3�ӻ�����D��ȷ��
��ѡCD��
��A��SO2�Ǽ��Է��ӣ�SO3�ǷǼ��Է��ӣ���A����
B����NH4+�У���ԭ�Ӻ���ԭ��֮�������λ����[Cu��NH3��4]2+�У�ͭ�͵�ԭ�Ӽ������λ������B��ȷ��
C��Ԫ�ص縺��Խ���ԭ�ӣ��������ӵ�����Խǿ����C��ȷ��
D��ԭ�Ӿ�����ԭ���Թ��ۼ���ϣ����ܴ��۵�ߡ�Ӳ�ȴ�D��ȷ��
��ѡA��
��1��MgԪ��ԭ�ӵ�3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬���������ͣ���һ�����ܸ���ͬ�������ڵ�Ԫ�أ��ʵ�һ������Al��Mg���ʴ�Ϊ������
��2�������д��ڴ�м���̼ԭ���ӻ���Ϊ2����ȡsp2�ӻ���������Cԭ�ӳ�4���������ӻ������Ϊ4����ȡsp3�ӻ���Oԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ����ʴ�Ϊ��C��N��O��
��3����ͼ��֪�����Ͻ�ľ����ṹ�У�Niԭ����ĿΪ1��Alԭ����ĿΪ8��
=1��ΪAlNi��ÿ��Niԭ����Χ��8��Alԭ�ӣ�ÿ��Alԭ����Χ��8��Niԭ�ӣ�
a���Ȼ�����ÿ����������Χ��6�������ӣ�ÿ����������Χ��6�������ӣ������ϣ�
b���Ȼ����ÿ���������Χ��8�������ӣ�ÿ����������Χ��8������ӣ����ϣ�
c��ʯӢΪ�ռ�������״�ṹ����ԭ����Χ��4����ԭ�ӣ���ԭ����Χ��2����ԭ�ӣ������ϣ�
d�����ʯΪ�ռ�������״�ṹ��ÿCԭ����Χ��4��Cԭ�ӣ������ϣ�
�ʴ�Ϊ��b��
��4����Ni��28��Ԫ�أ�������28�����ӣ���ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d84s2���ʴ�Ϊ��1s22s22p63s23p63d84s2��
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ͬ��������ԭ������ԭ��֮���γ��������ͼ��ʾ��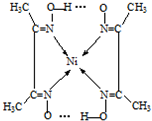 ��
��
�ʴ�Ϊ�� ��
��
B���ɼ۲���ӶԻ������ۿ�֪Cl03-������ԭ�ӵŵ��Ӷ�����
| 1 |
| 2 |
C����ԭ���������6�����ӣ��ͷ�ԭ��֮����6����ȫ��ͬ�ijɼ����Ӷԣ���C��ȷ��
D��SiF4��SO32- �Ŀռ乹�ͷֱ�Ϊ��������������Σ�������ԭ�Ӿ����õ���sp3�ӻ�����D��ȷ��
��ѡCD��
��A��SO2�Ǽ��Է��ӣ�SO3�ǷǼ��Է��ӣ���A����
B����NH4+�У���ԭ�Ӻ���ԭ��֮�������λ����[Cu��NH3��4]2+�У�ͭ�͵�ԭ�Ӽ������λ������B��ȷ��
C��Ԫ�ص縺��Խ���ԭ�ӣ��������ӵ�����Խǿ����C��ȷ��
D��ԭ�Ӿ�����ԭ���Թ��ۼ���ϣ����ܴ��۵�ߡ�Ӳ�ȴ�D��ȷ��
��ѡA��
��1��MgԪ��ԭ�ӵ�3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬���������ͣ���һ�����ܸ���ͬ�������ڵ�Ԫ�أ��ʵ�һ������Al��Mg���ʴ�Ϊ������
��2�������д��ڴ�м���̼ԭ���ӻ���Ϊ2����ȡsp2�ӻ���������Cԭ�ӳ�4���������ӻ������Ϊ4����ȡsp3�ӻ���Oԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ����ʴ�Ϊ��C��N��O��
��3����ͼ��֪�����Ͻ�ľ����ṹ�У�Niԭ����ĿΪ1��Alԭ����ĿΪ8��
| 1 |
| 8 |
a���Ȼ�����ÿ����������Χ��6�������ӣ�ÿ����������Χ��6�������ӣ������ϣ�
b���Ȼ����ÿ���������Χ��8�������ӣ�ÿ����������Χ��8������ӣ����ϣ�
c��ʯӢΪ�ռ�������״�ṹ����ԭ����Χ��4����ԭ�ӣ���ԭ����Χ��2����ԭ�ӣ������ϣ�
d�����ʯΪ�ռ�������״�ṹ��ÿCԭ����Χ��4��Cԭ�ӣ������ϣ�
�ʴ�Ϊ��b��
��4����Ni��28��Ԫ�أ�������28�����ӣ���ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d84s2���ʴ�Ϊ��1s22s22p63s23p63d84s2��
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ͬ��������ԭ������ԭ��֮���γ��������ͼ��ʾ��
 ��
���ʴ�Ϊ��
 ��
�����������⿼��֪ʶ���е����ܡ��ӻ��������ѧ������������Ų����ɵȣ��漰��֪ʶ��϶࣬��Ŀ�ۺ��Խ�ǿ���Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
��20��)
19-I(6��)������������ȷ����
| A��CS2ΪV�εļ��Է��� |
| B��Cl0�� 3 �Ŀռ乹��Ϊƽ�������� |
| C��SF6����6����ȫ��ͬ�ijɼ����Ӷ� |
| D��SiF4��SO2�� 3 ������ԭ�Ӿ�Ϊsp3�ӻ� |
(1)Niԭ�ӵĺ�������Ų�ʽΪ______________________________��
(2)Ni0��Fe0�ľ���ṹ���;����Ȼ��Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69 pm��78 pm�����۵�NiO ________ FeO(�<����>��)��
(3)Ni0������Ni��O����λ���ֱ�Ϊ_______________��_______________��
(4)����������(La)�γɵĺϽ���һ�����õĴ�����ϣ��侧���ṹʾ��ͼ������ͼ��ʾ���úϽ�Ļ�ѧʽΪ_______________��


(5)����ͪ뿳����ڼ���Ni2+����ϡ��ˮ�����У�����ͪ���Ni2+��Ӧ�������ʺ�ɫ��������ṹ������ͼ��ʾ��
�ٸýṹ�У�̼̼֮��Ĺ��ۼ�������
 ����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________���ڸýṹ�У�����֮������ۼ���ɴ���_______________��
 �۸ýṹ�У�̼ԭ�ӵ��ӻ����������_______________��
�۸ýṹ�У�̼ԭ�ӵ��ӻ����������_______________�� 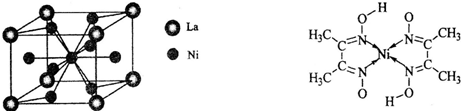


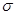 ����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��