��Ŀ����
��98%��Ũ����(�ܶ�Ϊ1.84 g/cm3,���ʵ���Ũ��Ϊ18.4mol/L)����1 mol/L��ϡH2SO4 100 mL�����ƹ����п����õ�����������
��100 mL��Ͳ����10 mL��Ͳ����50 mL�ձ�����������ƽ����100 mL����ƿ����ͷ�ιܡ��߲����� ��ʹ�õ��Ⱥ�˳��������ȷ����(����)
A���ܢۢߢݢ� B���ڢݢߢܢ�
C���ܢۢݢޢ� D���ڢۢߢݢ�
D

��ϰ��ϵ�д�
�����Ŀ
��20�֣�����98%��Ũ����(�ܶ�Ϊ1.84g��cm��3)���Ƴ�Ũ��Ϊ0.5mol��L��1��ϡ����500ml��
(1)ѡ�õ���Ҫ�����У�
�ٲ����������ձ�������Ͳ���ܽ�ͷ�ιܣ���____________��
(2)�뽫���и�����������ȷ��������ں����ϡ�
| A������Ͳ��ȡŨH2SO4 |
| B�������ߵ�ҡ�� |
| C���ý�ͷ�ιܼ�����ˮ���̶��� |
| D��ϴ���������� |
F������Һת������ƿ
�������ȷ��˳������Ϊ____________________________��
(3)��Ҫ�ش��������⣺
������Ũ��������Ϊ____________mL��
�����ʵ������15mL��20mL��50mL����ͲӦѡ��____________mL����Ͳ��ã���ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��ʹŨ��__________(ѡ�ƫ�ߡ�����ƫ�͡�������Ӱ�족������ͬ��)
����ת������ƿǰ�ձ���Һ��Ӧ____________�������ʹŨ��____________����ϴ���ձ��Ͳ�����2��3�Σ�ϴ��ҺҲҪת������ƿ�������ʹŨ��____________��
�ܶ���ʱ����ʹ��Һ��Һ����̶������У������ӻ�ʹŨ��____________��������ʹŨ��__________��
��1��д����ͼ����Ţ١������������ƣ�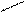
|



 I II III
I II III�� ���� �� �� ���� ��
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ���� ������������ţ�
��3�������ˮ�еĵ�Ӧ��ѡ��װ�� ����װ����ţ����� �� ����������װ�� ����װ����ţ����� ������
��4��������98%��Ũ����(�ܶ�Ϊ1.84g��cm��3)���Ƴ�Ũ��Ϊ0.5mol��L��1��ϡ����100mL��
�������������ձ������������ __________�� __________�� __________��
����ȡŨ��������Ϊ____________mL��
�����в�������������ҺŨ��ƫ�ߵ���
A ȡŨ����ʱ����
B ��Ũ���ᵹ����ϴ��װ�ã�����ϴ��Һ�����ձ���
C ���ձ���ϡ��Ũ���������ת��
D ����ʱ����
E �ߵ�ҡ�Ⱥ���Һ����ڿ̶��ߣ���δ��ˮ���̶���
