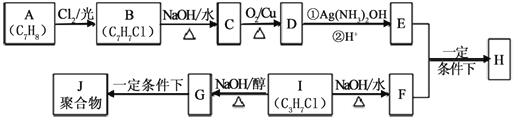
ЬтФПФкШн
ЁОЬтФПЁПЂёЁЂАДвЊЧѓЬюПеЃК
ЃЈ1ЃЉ32 g CH4ЃЌЦфФІЖћжЪСПЮЊ_______ЃЌдМКЌга____ФІЕчзгЃЌдкБъзМзДПіЯТЫљеМЕФЬхЛ§дМЮЊ____LЁЃ
ЃЈ2ЃЉЕШЬхЛ§ЕШЮяжЪЕФСПХЈЖШЕФCa(OH)2ШмвКгыNaHCO3ШмвКЛьКЯЃЌРызгЗНГЬЪНЮЊЃК______ЁЃ
ЃЈ3ЃЉH++HCO3-ЃНH2O+CO2ЁќЖдгІЕФвЛИіЛЏбЇЗНГЬЪН________________________ЁЃ
ЃЈ4ЃЉжЦБИЧтбѕЛЏЬњНКЬхЕФРызгЗНГЬЪН________________ЁЃ
ЂђЁЂЯжгавдЯТЮяжЪЃК
ЂйNaClЙЬЬх ЂквКЬЌSO3 ЂлвКЬЌЕФДзЫс ЂмЙЏ ЂнBaSO4ЙЬЬх ЂоесЬЧ(C12H22O11) ЂпОЦОЋ(C2H5OH) ЂрШлЛЏЕФKNO3 ЂсбЮЫсЁЃЧыЛиД№ЯТСаЮЪЬт(ЬюађКХ)ЁЃ
ЃЈ1ЃЉвдЩЯЮяжЪжаФмЕМЕчЕФЪЧ__________________ЁЃ
ЃЈ2ЃЉвдЩЯЮяжЪЪєгкЕчНтжЪЕФЪЧ_________________ЁЃ
ЃЈ3ЃЉвдЩЯЮяжЪжаЪєгкЗЧЕчНтжЪЕФЪЧ______________ЁЃ
ЁОД№АИЁП16g/mol 20 44.8 Ca2++OHЃ+HCO3ЃЃНCaCO3Ё§+H2O HCl+NaHCO3ЃНNaCl+CO2Ёќ+H2O Fe3++3H2O![]() Fe(OH)3(НКЬх)+3H+ ЂмЂрЂс ЂйЂлЂнЂр ЂкЂоЂп
Fe(OH)3(НКЬх)+3H+ ЂмЂрЂс ЂйЂлЂнЂр ЂкЂоЂп
ЁОНтЮіЁП
ЂёЁЂЃЈ1ЃЉМзЭщЕФЯрЖдЗжзгжЪСПЪЧ16ЃЌдђМзЭщЕФФІЖћжЪСПЪЧ16g/molЃЌ32 g CH4ЕФЮяжЪЕФСПЪЧ32gЁТ16g/molЃН2molЃЌ1ЗжзгМзЭщКЌга10ИіЕчзгЃЌвђДЫКЌга20molЕчзгЁЃИљОнV=nVmПЩжЊдкБъзМзДПіЯТЫљеМЕФЬхЛ§дМЮЊ2molЁС22.4L/molЃН44.8LЁЃ
ЃЈ2ЃЉЕШЬхЛ§ЕШЮяжЪЕФСПХЈЖШЕФCa(OH)2ШмвКгыNaHCO3ШмвКЛьКЯЗДгІЩњГЩЬМЫсИЦЁЂЧтбѕЛЏФЦКЭЫЎЃЌЗДгІЕФРызгЗНГЬЪНЮЊCa2++OHЃ+HCO3ЃЃНCaCO3Ё§+H2OЁЃ
ЃЈ3ЃЉH++HCO3-ЃНH2O+CO2ЁќЖдгІЕФвЛИіЛЏбЇЗНГЬЪНПЩвдЪЧHCl+NaHCO3ЃНNaCl+CO2Ёќ+H2OЁЃ
ЃЈ4ЃЉБЅКЭТШЛЏЬњШмвКЕЮШыЗаЫЎжаМЬајМгШШвЛЖЮЪБМфКѓМДПЩЕУЕНЧтбѕЛЏЬњНКЬхЃЌдђжЦБИЧтбѕЛЏЬњНКЬхЕФРызгЗНГЬЪНЮЊFe3++3H2O![]() Fe(OH)3(НКЬх)+3H+ЁЃ
Fe(OH)3(НКЬх)+3H+ЁЃ
ЂђЁЂШмгкЫЎЛђШлШкзДЬЌЯТФмЙЛЕМЕчЕФЛЏКЯЮяЪЧЕчНтжЪЃЌШмгкЫЎКЭдкШлШкзДЬЌЯТОљВЛФмЕМЕчЕФЛЏКЯЮяЪЧЗЧЕчНтжЪЃЌКЌгаздгЩвЦЖЏЕчзгЛђРызгЕФЮяжЪПЩвдЕМЕчЁЃ
ЂйNaClЙЬЬхВЛЕМЕчЃЌЪЧЕчНтжЪЃЛ
ЂквКЬЌSO3ВЛЕМЕчЃЌЪЧЗЧЕчНтжЪЃЛ
ЂлвКЬЌЕФДзЫсВЛЕМЕчЃЌЪЧЕчНтжЪЃЛ
ЂмЙЏЪЧН№ЪєЃЌФмЕМЕчЃЌВЛЪЧЕчНтжЪвВВЛЪЧЗЧЕчНтжЪЃЛ
ЂнBaSO4ЙЬЬхВЛЕМЕчЃЌЪЧЕчНтжЪЃЛ
ЂоесЬЧ(C12H22O11)ВЛЕМЕчЃЌЪЧЗЧЕчНтжЪЃЛ
ЂпОЦОЋ(C2H5OH)ВЛЕМЕчЃЌЪЧЗЧЕчНтжЪЃЛ
ЂрШлЛЏЕФKNO3ФмЕМЕчЃЌЪЧЕчНтжЪЃЛ
ЂсбЮЫсФмЕМЕчЃЌЪєгкЛьКЯЮяЃЌВЛЪЧЕчНтжЪвВВЛЪЧЗЧЕчНтжЪЁЃдђ
ЃЈ1ЃЉвдЩЯЮяжЪжаФмЕМЕчЕФЪЧЂмЂрЂсЁЃ
ЃЈ2ЃЉвдЩЯЮяжЪЪєгкЕчНтжЪЕФЪЧЂйЂлЂнЂрЁЃ
ЃЈ3ЃЉвдЩЯЮяжЪжаЪєгкЗЧЕчНтжЪЕФЪЧЂкЂоЂпЁЃ

ЁОЬтФПЁПСђЛЏМюЗЈЪЧЙЄвЕЩЯжЦБИNa2S2O3ЕФЗНЗЈжЎвЛЃЌЗДгІдРэЮЊЃК2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2ЃЈИУЗДгІЁїHЃО0ЃЉЁЃФГбаОПаЁзщдкЪЕбщЪвгУСђЛЏМюЗЈжЦБИNa2S2O3ЁЄ5H2OСїГЬШчЯТЁЃ

ЃЈ1ЃЉЮќСђзАжУШчЭМЫљЪОЁЃ

ЂйзАжУBЕФзїгУЪЧМьбщзАжУAжаSO2ЕФЮќЪеаЇТЪЃЌBжаЪдМСЪЧ_____________ЃЌБэУїSO2ЮќЪеаЇТЪЕЭЕФЪЕбщЯжЯѓЪЧBжаШмвК______________ЁЃ
ЂкЮЊСЫЪЙSO2ОЁПЩФмЮќЪеЭъШЋЃЌдкВЛИФБфAжаШмвКХЈЖШЁЂЬхЛ§ЕФЬѕМўЯТЃЌГ§СЫМАЪБНСАшЗДгІЮяЭтЃЌЛЙПЩВЩШЁЕФКЯРэДыЪЉЪЧ______________ЁЃЃЈШЮаДвЛЬѕЃЉ
ЃЈ2ЃЉМйЩшБОЪЕбщЫљгУЕФNa2CO3КЌЩйСПNaClЁЂNaOHЃЌЩшМЦЪЕбщЗНАИНјааМьбщЁЃЃЈЪвЮТЪБCaCO3БЅКЭШмвКЕФpHЃН10.2ЃЉЃЌЯобЁЪдМСМАвЧЦїЃКЯЁЯѕЫсЁЂAgNO3ШмвКЁЂCaCl2ШмвКЁЂЗгЬЊШмвКЁЂеєСѓЫЎЁЂpHМЦЁЂЩеБЁЂЪдЙмЁЂЕЮЙм
ађКХ | ЪЕбщВйзї | дЄЦкЯжЯѓ | НсТл |
Ђй | ШЁЩйСПбљЦЗгкЪдЙмжаЃЌМгШыЪЪСПеєСѓЫЎЃЌГфЗжеёЕДШмНтЃЌ____________ ЁЃ | гаАзЩЋГСЕэЩњГЩ | бљЦЗКЌNaCl |
Ђк | СэШЁЩйСПбљЦЗгкЩеБжаЃЌМгШыЪЪСПеєСѓЫЎЃЌГфЗжНСАшШмНтЃЌ_______ЁЃ | гыАзЩЋГСЕэЩњГЩЃЌЩЯВуЧхвКpH>10.2 | бљЦЗКЌNaOH |
ЃЈ3ЃЉNa2S2O3ШмвКЪЧЖЈСПЪЕбщжаЕФГЃгУЪдМСЃЌВтЖЈЦфХЈЖШЕФЙ§ГЬШчЯТЃК
ЕквЛВНЃКзМШЗГЦШЁa g KIO3(ЯрЖдЗжзгжЪСПЃК214)ЙЬЬхХфГЩШмвКЃЌ
ЕкЖўВНЃКМгШыЙ§СПKIЙЬЬхКЭH2SO4ШмвКЃЌЕЮМгжИЪОМСЃЌ
ЕкШ§ВНЃКгУNa2S2O3ШмвКЕЮЖЈжСжеЕуЃЌЯћКФNa2S2O3ШмвКЕФЬхЛ§ЮЊv mLЁЃдђc(Na2S2O3)ЃН______molЁЄL-1ЁЃЃЈжЛСаГіЫуЪНЃЌВЛзїдЫЫуЃЉ
вбжЊЃКIO3-+I-+6H+=3I2+3H2O 2S2O32-+I2=S4O62-+2I-ЁЃМзЭЌбЇЪЂзАNa2S2O3ШмвКжЎЧАЮДШѓЯДЃЌетбљВтЕУЕФNa2S2O3ЕФХЈЖШПЩФм________ЃЈЬюЁАЮогАЯьЁБЁЂЁАЦЋЕЭЁБЛђЁАЦЋИпЁБ)ЃЛввЭЌбЇЕквЛВНКЭЕкЖўВНЕФВйзїЖМКмЙцЗЖЃЌЕкШ§ВНЕЮЫйЬЋТ§ЃЌетбљВтЕУЕФNa2S2O3ЕФХЈЖШПЩФм________(ЬюЁАЮогАЯьЁБЁЂЁА ЦЋЕЭЁБЛђЁАЦЋИпЁБ)ЁЃ