��Ŀ����
��14�֣�������һ�������Դ���⡣����Ӧ�ú��磬�������ȼҵ��ԭ�ϡ�
��1�����ᴿ���ʳ������20%��NaCl��Һ��Ӧѡ�õ�������__________��ѡ����ţ���
a. ��ƿ b. ����ƿ c. ��Ͳ d. ��ͷ�ι�
��2����ⱥ��ʳ��ˮ���ø�Ĥ���ۻ�����Ĥ���ۡ�ͼ7Ϊ�����ӽ���Ĥ���ۣ�ֻ����������ͨ����ʾ��ͼ��
�����ж�EΪ_________����
���Ƶõ��ռ���Һ��_______��������ĸ��������
���Ƶõ��ռ���Һ����������NaCl���������к���Cl���ľ�������ǣ�______________________________________________________________________��
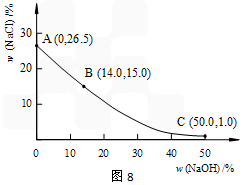
��3��20��ʱ�Ƶ��ռ���Һ�У�����NaOH�����ı仯��NaCl�ﵽ����״̬ʱ���������������ı仯������ͼ������20��ʱ����������B����ʾ����Һ���ɲ���______________
��������NaCl�ĺ������ﵽ�ᴿĿ�ģ���������_________________________________________________________________��
��4�����ⶨij�����ռ���Һ��Ʒ����֪�ܶ�Ϊ��g��cm��3����NaOH�ĺ������ɲ��õķ�����___________��
��1��cd��2�֣�
��2����������2�֣���L��2�֣���ȡ���������������ᣨ����������ټ���AgNO3��Һ���а�ɫ������2�֣�
��3�������ᾧ�����NaOH����Ũ������2�֣�
��Һ��NaOH����Խ�ߣ�NaCl����Խ�ͣ���C�㣬��NaOHΪ50%ʱ��NaCl����1%�������ԡ�����2�֣�
��4�����кͣ��ζ�����2�֣�
����

��I������ѡ���⣨6�֣�
��ѧ���ѧ����������ᡢ����������أ�������������ȷ����__________��
| A�������Ҵ�����(����������һ�������Ҵ�)���������ܽ��ͻ�����β�����к������ŷ� |
| B����ҵ����ʯ�����úȼ�պ��γɵ��������������������Ƶ�ʯ�� |
C��Ϊ����Ч�ķ�չ�����Դ�����õ��ˮ�ķ����� ���Ʊ�H2 ���Ʊ�H2 |
| D������ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� |
��II����14�֣�
��嫵ĺ�����һ�������Դ���⣬�̲��ŷ��ĵĿ�����DZ���Ļ�ѧ��Դ����ͼ�Ǻ�ˮ�ӹ���ʾ��ͼ��������ͼ�ش����⡣

��1����ˮ��������ͨ�����õ��Ʊ���ˮ�ķ����� ��д�����֣���
��2����ͼ�Ǵ�Ũ����ˮ����ȡ��
 ������ͼ��д����ͼ�Т٢ڵĻ�ѧʽ���� ���� ���������з����Ļ�ѧ��Ӧ����ʽΪ ��
������ͼ��д����ͼ�Т٢ڵĻ�ѧʽ���� ���� ���������з����Ļ�ѧ��Ӧ����ʽΪ ��
��3���Ʊ�����þ��ͨ��������ڵ�MgC12��������MgO����ԭ���� ��
��4��ʳ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ��ȼҵ����ͨ����ⱥ��ʳ��ˮ���Ʊ�NaOH��H2��C12����ˮ�еõ��Ĵ�������������һЩ���ʣ��������һЩ��ѧ�Լ���ʹ���ʳ��������������ˮ������������ӽ���������ԭ���� �����ʳ��ˮ�����ӽ���Ĥ�����н��У����ӽ���Ĥ�������� ��
��5���ྦྷ����Ҫ����SiHCl3��ԭ�����������丱����SiCl4��ת��ΪSiHCl3��ѭ��ʹ�á�һ�������£���20L�����ܱ������еķ�Ӧ��3 SiCl4��g��+2 H2��g��+Si��g��
 4 SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl��
4 SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl�� ����Ϊ kg��
����Ϊ kg�� 



 4
SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl������Ϊ
kg��
4
SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl������Ϊ
kg��