��Ŀ����
��1����ȼ�������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
��2��ȡһ�����ı�����ˮ��CaCO3��ĩ��ϣ��۲쵽�������ݣ���ˮ�Ļ���ɫ��ȥ����ֹ��ȡ�ϲ������Һ�ķݣ��ֱ��������ʵ�飺
�� һ�ݵμ����ᣬ���������� ��
��
�� һ�ݵμ�NaOH��Һ�����ְ�ɫ����
�� һ���þƾ��Ƽ��ȳ��ְ�ɫ����
�� ����ɫ����������ķ���Һ������������ɫ
������ʵ�������Ʋ�ó�����Һ����Ҫ������ ��
ʵ��ٿ����� ����ʽ��ʾ��
������ˮ��CaCO3��ĩ������Ӧ�Ļ�ѧ����ʽ ��

��2��ȡһ�����ı�����ˮ��CaCO3��ĩ��ϣ��۲쵽�������ݣ���ˮ�Ļ���ɫ��ȥ����ֹ��ȡ�ϲ������Һ�ķݣ��ֱ��������ʵ�飺
�� һ�ݵμ����ᣬ����������
 ��
���� һ�ݵμ�NaOH��Һ�����ְ�ɫ����
�� һ���þƾ��Ƽ��ȳ��ְ�ɫ����
�� ����ɫ����������ķ���Һ������������ɫ
������ʵ�������Ʋ�ó�����Һ����Ҫ������ ��
ʵ��ٿ����� ����ʽ��ʾ��

������ˮ��CaCO3��ĩ������Ӧ�Ļ�ѧ����ʽ ��
29����1�� b ��
��2����Ҫ������ Ca(HCO3)2 CaCl2 HClO ��
ʵ��ٿ����� Ca(HCO3)2 + 2HCl = CaCl2 + CO2 + 2H2O ��ʾ��
��ѧ����ʽ 2CaCO3 + 2Cl2 + 2H2O = Ca(HCO3)2 + CaCl2 + 2HClO ��
��2����Ҫ������ Ca(HCO3)2 CaCl2 HClO ��
ʵ��ٿ����� Ca(HCO3)2 + 2HCl = CaCl2 + CO2 + 2H2O ��ʾ��
��ѧ����ʽ 2CaCO3 + 2Cl2 + 2H2O = Ca(HCO3)2 + CaCl2 + 2HClO ��
��

��ϰ��ϵ�д�
�����Ŀ
 ���衣
���衣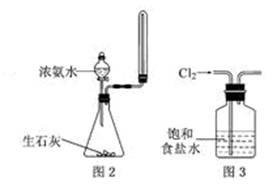

 ���ױ�Ʒ
���ױ�Ʒ ������IJ����У�������ȵ��ǣ� ��
������IJ����У�������ȵ��ǣ� ��