��Ŀ����
����˵����ȷ����
| A��NaHCO3��Na2CO3�����Һ�У�һ���� c(Na��)��c(H��)��c(OH��)��c(HCO3-) ��c(CO32-) |
| B��Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��pH�ɴ�С����˳��Ϊ NaOH>Na2CO3>NaHSO4>(NH4)2SO4 |
| C�������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С |
| D��pH=3�������������Һ��c(SO42��)��c(CH3COO��)֮��Ϊ1��2 |
D
���������A������ȷ������غ�ӦΪ��c(Na��)��c(H��)��c(OH��)��c(HCO3-) ��2c(CO32-)��B������ȷ��NaOH��ǿ����ʣ���ȫ���룬c��OH�� ��=0.1mol��L��1������pH=13��Na2CO3��ǿ�������Σ�ˮ��Һ���ʼ��ԣ�pHС��13��(NH4)2SO4��ǿ�������Σ�ˮ�����Һ�����ԣ�1��pHֵ��7��NaHSO4��ǿ����ʽ�Σ���ˮ����ȫ����������ӡ���������ӡ������ӣ�����C��H����=0.1mol��L��1������pH=1������pH�ɴ�С����˳��ΪNaOH>Na2CO3>(NH4)2SO4>NaHSO4��C������ȷ���ڱ���������μ�ˮ���������ܽⲢ���룬����̶�����������Ũ�����������ڣ���������ϡ�ͣ�����Ũ�ȼ�С������Һ�ĵ����Խ���С������̶�����Һ��ϡ�ͣ�Խ��Խ����Һ��pH��������С��D����ȷ�� pH=3��H2SO4��c(H�� )=0.001mol/L,c(SO42�D )=0.0005mol/L,C(CH3COO�D)=0.001mol/L��c(SO42��)��c(CH3COO��)֮��Ϊ1��2��ѡD��

��ϰ��ϵ�д�
�����Ŀ
 ����Һ�У�K����I����Cl����NO3��
����Һ�У�K����I����Cl����NO3��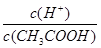 ����
����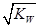 mol/L
mol/L