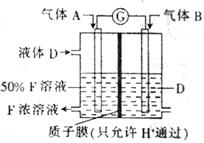
��Ŀ����
��9�֣���һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش��������⣬
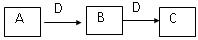
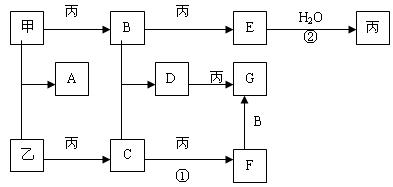
��֪A��B��C�о�����ͬһ��Ԫ�أ�DΪ�ǽ������ʣ���ʹ���л��ǵ�ľ����ȼ��
��1����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��д��A���ʵĵ���ʽ ��
��C��ˮ��Ӧ���õ���Һ�����ԣ��˷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ����
��2����AΪ���嵥�ʣ�CΪ����ɫ���壬����C���еĻ�ѧ���� ��
A��ˮ��Ӧ�����ӷ���ʽΪ ��
��3����AΪ�ճ�����������������������A��C��Ӧ����һ����ˮ����ζ�����ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ , ��Ӧ����Ϊ ��Ӧ��

��֪A��B��C�о�����ͬһ��Ԫ�أ�DΪ�ǽ������ʣ���ʹ���л��ǵ�ľ����ȼ��
��1����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��д��A���ʵĵ���ʽ ��
��C��ˮ��Ӧ���õ���Һ�����ԣ��˷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ����
��2����AΪ���嵥�ʣ�CΪ����ɫ���壬����C���еĻ�ѧ���� ��
A��ˮ��Ӧ�����ӷ���ʽΪ ��
��3����AΪ�ճ�����������������������A��C��Ӧ����һ����ˮ����ζ�����ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ , ��Ӧ����Ϊ ��Ӧ��
��9�֣�
�� ������1��2��
������1��2��
�����Ӽ����ۼ��� 2Na+2H2O=2Na++2OH- +H2��
�� CH3CH2OH+CH3COOH��CH3COOCH2CH3+H2O ����
��
 ������1��2��
������1��2�������Ӽ����ۼ��� 2Na+2H2O=2Na++2OH- +H2��
�� CH3CH2OH+CH3COOH��CH3COOCH2CH3+H2O ����
��

��ϰ��ϵ�д�
 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д�
�����Ŀ