��Ŀ����
��13�֣������к��зḻ�ĵ�Ԫ�أ���I����ʽ���ڣ���ʵ��������ȡ����������£�
��1��ʵ������۵������� ��������Ҫ��������Ϊ ��
��2����ȡ��Ĺ����У��ɹ�ѡ����л��Լ��� ������ţ���
A���ƾ����е�78�棩 B�����Ȼ�̼���е�77�棩
C�����ͣ��е�290�棩 D�������е�80�棩
��3��Ϊʹ�Ӻ����л���Һ����ȡ�Ⲣ�����ܼ�˳�����У�����ˮԡ������������ͼ��ʾ������ָ��ͼ��ʵ��װ���д���֮��
�� _ ___���� ___
��4��ʵ����ʹ��ˮԡ��ԭ���� ��������ۼ��� �����������ƣ��С�
��5���ڴ���Ӧ��ѧ����ʽ
��1����ȡ��Һ ����Һ©�� ��ÿ��1�֣���2�֣�
��2��B D ��2�֣�
��3���� ȱʯ���� �� �¶ȼƲ嵽Һ���� �� �����ܽ���ˮ����ߵ�
��ÿ��1�֣���3�֣�
��4���л��ܼ��е�ϵͣ������¶Ȳ��ܹ��ߣ�������������������ܣ�
������ƿ ��ÿ��1�֣���2�֣�
����:

 ��У����ϵ�д�
��У����ϵ�д�




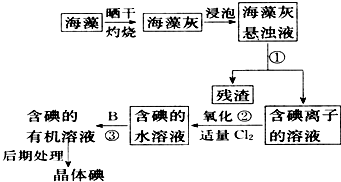 ����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ�������ӵ���ʽ���ڣ�ʵ���дӺ�����ȡ���������ͼ��ʾ��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ�������ӵ���ʽ���ڣ�ʵ���дӺ�����ȡ���������ͼ��ʾ��