��Ŀ����
����Ŀ���о�CO2�ں����е�ת�ƺ���,�ǵ������ѧ�о���ǰ������
(1)���ں�ˮ��CO2��Ҫ��4����̼��ʽ����,����HC![]() ռ95%,д����ˮ��̼������ˮ������ӷ���ʽ____________________________��
ռ95%,д����ˮ��̼������ˮ������ӷ���ʽ____________________________��
(2)�ں���̼ѭ����,ͨ����ͼ��ʾ��;����̼��

��д���ƻ����õ����ӷ���ʽ����������ͼ�еļ�ͷ�������ַ�Ӧ���������_____________________________________��
��ͬλ��ʾ�ٷ�֤ʵ��������ͷų���O2ֻ����H2O,��18O������ʵĹ�����õĻ�ѧ����ʽ����,���䲹������:____��____![]() (CH2O)x��x18O2��xH2O
(CH2O)x��x18O2��xH2O
(3)��ˮ���ܽ���̼ռ��ˮ��̼��95%����,��ȷ�������о�����̼ѭ���Ļ���,�����ܽ���̼,�ɲ������·���:
������CO2����N2���ữ��ĺ�ˮ�д���CO2���ü�Һ����(װ��ʾ��ͼ����),�����߿��е�װ�ò�����������������Լ���������ܽ�����ϴ��ƿ������ͼ����ữ��������ָ�����õ��ᣩ��
____
ָ������ʢװNaOH��Һ��ϴ��ƿ�����÷ֱ�Ϊ��
______________________________��______________________________��

�ڵζ���������Һ���յ���̼ת��ΪNaHCO3,����HCl��Һ�ζ�,�Ӷ���ú�ˮ���ܽ���̼��Ũ�ȡ�
(4)������ͼ��ʾװ�ôӺ�ˮ����ȡCO2,�����ڼ��ٻ����������庬����

��a�ҷ����ĵ缫��Ӧ���ɵ������뺣ˮ�е�HC![]() ������Ӧ�Ļ�ѧ����ʽΪ_______________,�÷�Ӧ��_____�ҽ��У��a������b����c������
������Ӧ�Ļ�ѧ����ʽΪ_______________,�÷�Ӧ��_____�ҽ��У��a������b����c������
��b���ų��ĺ�ˮ����ֱ���Żش�Ӧ�ø�װ����____�Ҳ��������ʴ������a������b����c����,ʹ��ˮ�ӽ����Ժ��Żش�
���𰸡� HC![]() ��H2O
��H2O ![]() H2CO3��OH�� Ca2����2HC
H2CO3��OH�� Ca2����2HC![]() CaCO3����CO2����H2O xCO2 2x
CaCO3����CO2����H2O xCO2 2x![]() O
O  ��һ��ϴ��ƿ�������պ�ˮ�е���̼���ɵĶ�����̼ �ڶ���ϴ��ƿ���ڷ�ֹ�����еĶ�����̼�����һ��ϴ��ƿ��ʹ�ò�����ȷ HC
��һ��ϴ��ƿ�������պ�ˮ�е���̼���ɵĶ�����̼ �ڶ���ϴ��ƿ���ڷ�ֹ�����еĶ�����̼�����һ��ϴ��ƿ��ʹ�ò�����ȷ HC![]() +H+ CO2��+H2O b c
+H+ CO2��+H2O b c
����������1����ˮ��̼������ˮ������ӷ���ʽ��HC![]() ��H2O
��H2O ![]() H2CO3��OH����(2)��д���ƻ����õ����ӷ���ʽ����Ӧ���к���̼�������������Ϊ̼��ƺ�CO2������Ԫ���غ��Լ�����غ�ó�����ʽΪ��2HCO3��+Ca2��=CaCO3��+CO2��+H2O���ڹ�����ò�����O2��Դ��ˮ����ˮ�е���ԭ�Ӳ���ʾ�ٷ����Ϊ18O������Ԫ�������غ㣬Ӧ��ҪCO2��H218O���ʴ�Ϊ�� xCO2��2x
H2CO3��OH����(2)��д���ƻ����õ����ӷ���ʽ����Ӧ���к���̼�������������Ϊ̼��ƺ�CO2������Ԫ���غ��Լ�����غ�ó�����ʽΪ��2HCO3��+Ca2��=CaCO3��+CO2��+H2O���ڹ�����ò�����O2��Դ��ˮ����ˮ�е���ԭ�Ӳ���ʾ�ٷ����Ϊ18O������Ԫ�������غ㣬Ӧ��ҪCO2��H218O���ʴ�Ϊ�� xCO2��2x![]() O����3���ٿ���ʹ��ϡ�����ữ��ˮ�����÷�Һ©���μӣ����ܽ������̹ܳ�������װ��Ϊ��
O����3���ٿ���ʹ��ϡ�����ữ��ˮ�����÷�Һ©���μӣ����ܽ������̹ܳ�������װ��Ϊ��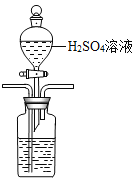 ������ʢװNaOH��Һ��ϴ��ƿ�����÷ֱ�Ϊ����һ��ϴ��ƿ�������պ�ˮ�е���̼���ɵĶ�����̼�� �ڶ���ϴ��ƿ���ڷ�ֹ�����еĶ�����̼�����һ��ϴ��ƿ��ʹ�ò�����ȷ��(4)��a�ң�2H2O-4e-=4H++O2����������ͨ�������ӽ���Ĥ����b�ң�������Ӧ��H++HCO3-=CO2��+H2O�����ø�װ�ò��������ʴ���b���ų��ĺ�ˮ���ϸ���Żش��������ϸ�ķ����ǣ�c�ң�2H2O+2e-=2OH-+H2������c���ų��ļ�Һ����b���ų������Ժ�ˮ������װ����ں�ˮ��pH��
������ʢװNaOH��Һ��ϴ��ƿ�����÷ֱ�Ϊ����һ��ϴ��ƿ�������պ�ˮ�е���̼���ɵĶ�����̼�� �ڶ���ϴ��ƿ���ڷ�ֹ�����еĶ�����̼�����һ��ϴ��ƿ��ʹ�ò�����ȷ��(4)��a�ң�2H2O-4e-=4H++O2����������ͨ�������ӽ���Ĥ����b�ң�������Ӧ��H++HCO3-=CO2��+H2O�����ø�װ�ò��������ʴ���b���ų��ĺ�ˮ���ϸ���Żش��������ϸ�ķ����ǣ�c�ң�2H2O+2e-=2OH-+H2������c���ų��ļ�Һ����b���ų������Ժ�ˮ������װ����ں�ˮ��pH��

����Ŀ����ͼ��ʾװ��������ʵ�飬�����ռ������������Լ��������۾���ȷ����

ѡ�� | ���� | �Լ� | ���� | ���� |
A | NH3 | ��̪��Һ | ��Һ���ɫ | NH3��ˮ��Һ�ʼ��� |
B | C12 | ��ɫʯ����Һ | ��Һ�ȱ�����ɫ | ��ˮ�����Ժ�Ư���� |
C | HCl | ��������Һ | ���ɰ�ɫ���� | C1�ķǽ����Ա�Siǿ |
D | Y | ����KMnO4��Һ | ��Һ��ɫ | Yһ����SO2 |
A. A B. B C. C D. D