��Ŀ����
������ЧӦ���Ǹ����� ��������仯����о��Ļ�������֮һ��CO2�����ڴ������о������Ⱥ��ȵĹ��ܣ�����Ҫ���������塣
��������仯����о��Ļ�������֮һ��CO2�����ڴ������о������Ⱥ��ȵĹ��ܣ�����Ҫ���������塣
��1�����д�ʩ�У������ڽ��ʹ�����CO2Ũ�ȵ��� ������ĸ��ţ���
a�����ý��ܼ��������ٻ�ʯȼ�ϵ�����
b�������������������У���������̼������
c������̫���ܡ����ܵ�������Դ�����ʯȼ�� ��
��2��CH4����һ����Ҫ���������壬l�˼�����ȫȼ������Һ̬ˮ�Ͷ�����̼���ų�55.64 kJ�������������ȼ������ ��
��3���ᰢ���γ���Ҫ���ɷ����е�SOx��NOx��ɵġ�ͨ��SO2��Br2��H2O�Ķ�����Ӧ�ڵ����в����ĵ����仯������ȷ�ⶨ������SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ����������ͻ�ԭ��������ʵ���֮��Ϊ ��
��4��ij���Ṥ���Ի�����Ϊԭ���������ᡣ
��һ��ȼ�ջ�����Ļ�ѧ����ʽΪ ��
�ڶ��εķ�Ӧԭ����2SO2��g��+O2��g��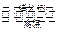 2SO3��g�����������豸������Ϊ ��������������ijһʱ��ȡ��������SO2��O2��SO3��Ũ�ȷֱ�Ϊ
2SO3��g�����������豸������Ϊ ��������������ijһʱ��ȡ��������SO2��O2��SO3��Ũ�ȷֱ�Ϊ
2mol��L-1��2mol��L-1��3mol��L-1������Ӧ�ﵽƽ��ʱ�����ܴ��ڵ������� ������ĸ��ţ�
a. SO2Ϊ5mol��L-1��O2Ϊ3.5mol��L-1
b. SO2Ϊ3mol��L-1
c.SO2��SO3��Ϊ2.5mol��L-1
d.SO3Ϊ5mol��L-1
 ��������仯����о��Ļ�������֮һ��CO2�����ڴ������о������Ⱥ��ȵĹ��ܣ�����Ҫ���������塣
��������仯����о��Ļ�������֮һ��CO2�����ڴ������о������Ⱥ��ȵĹ��ܣ�����Ҫ���������塣��1�����д�ʩ�У������ڽ��ʹ�����CO2Ũ�ȵ��� ������ĸ��ţ���
a�����ý��ܼ��������ٻ�ʯȼ�ϵ�����
b�������������������У���������̼������
c������̫���ܡ����ܵ�������Դ�����ʯȼ�� ��
��2��CH4����һ����Ҫ���������壬l�˼�����ȫȼ������Һ̬ˮ�Ͷ�����̼���ų�55.64 kJ�������������ȼ������ ��
��3���ᰢ���γ���Ҫ���ɷ����е�SOx��NOx��ɵġ�ͨ��SO2��Br2��H2O�Ķ�����Ӧ�ڵ����в����ĵ����仯������ȷ�ⶨ������SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ����������ͻ�ԭ��������ʵ���֮��Ϊ ��
��4��ij���Ṥ���Ի�����Ϊԭ���������ᡣ
��һ��ȼ�ջ�����Ļ�ѧ����ʽΪ ��
�ڶ��εķ�Ӧԭ����2SO2��g��+O2��g��
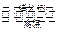 2SO3��g�����������豸������Ϊ ��������������ijһʱ��ȡ��������SO2��O2��SO3��Ũ�ȷֱ�Ϊ
2SO3��g�����������豸������Ϊ ��������������ijһʱ��ȡ��������SO2��O2��SO3��Ũ�ȷֱ�Ϊ2mol��L-1��2mol��L-1��3mol��L-1������Ӧ�ﵽƽ��ʱ�����ܴ��ڵ������� ������ĸ��ţ�
a. SO2Ϊ5mol��L-1��O2Ϊ3.5mol��L-1
b. SO2Ϊ3mol��L-1
c.SO2��SO3��Ϊ2.5mol��L-1
d.SO3Ϊ5mol��L-1
��1��a��b��c�������1�֣�
��2��890.24kJ��mol-1
��3��SO2+Br2+2H2O=2HBr+H2SO4 1��2
��4��4FeS2+11O2 2Fe2O3+8SO2 �Ӵ��� b��c�������1�֣�������÷֣�
2Fe2O3+8SO2 �Ӵ��� b��c�������1�֣�������÷֣�
��2��890.24kJ��mol-1
��3��SO2+Br2+2H2O=2HBr+H2SO4 1��2
��4��4FeS2+11O2
 2Fe2O3+8SO2 �Ӵ��� b��c�������1�֣�������÷֣�
2Fe2O3+8SO2 �Ӵ��� b��c�������1�֣�������÷֣���

��ϰ��ϵ�д�
�����Ŀ
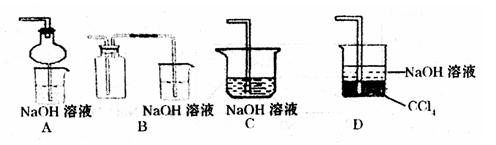
 ��ú��ȼ�� ��ȼ�ű��ڢ۹�ҵ�����������ŷŢ�����β��
��ú��ȼ�� ��ȼ�ű��ڢ۹�ҵ�����������ŷŢ�����β��