��Ŀ����
��һ�ݹ��壬������NaOH��NaHCO3��Na2CO3�е�һ�ֻ�������ɣ����÷ֲ��ζ�����˫ָʾ�������ⶨ����ɺ���ֵ������������䲽�����£�
��1����ȡw g���������ƿ�У�����30 mL����ˮʹ֮��ȫ�ܽ⣬�ȼ���2��3�η�̪��ָʾ����
��2����0.1 mol��L-1�ı�������еζ�������ƿ����Һ��__________ɫ���__________ɫʱ��ֹͣ�ζ�����ʱ����0.1 mol��L-1�ı�����V1 mL�����ⲽ�����У�Ӧ��__________�ֿ��ƻ�����__________�ֳ���ƿ������__________������ע��______________________________��
��3��������ƿ�м�2��3�μ�����ָʾ����������0.1 mol��L-1������ζ�������Һ��__________ɫ��Ϊ__________ɫʱֹͣ�ζ�����ʱ����0.1 mol��L-1������V2 mL��
��4����V2��V1ʱ����ԭ������ɿ���Ϊ__________�����ţ���
E.ֻ����NaHCO3
��1����ȡw g���������ƿ�У�����30 mL����ˮʹ֮��ȫ�ܽ⣬�ȼ���2��3�η�̪��ָʾ����
��2����0.1 mol��L-1�ı�������еζ�������ƿ����Һ��__________ɫ���__________ɫʱ��ֹͣ�ζ�����ʱ����0.1 mol��L-1�ı�����V1 mL�����ⲽ�����У�Ӧ��__________�ֿ��ƻ�����__________�ֳ���ƿ������__________������ע��______________________________��
��3��������ƿ�м�2��3�μ�����ָʾ����������0.1 mol��L-1������ζ�������Һ��__________ɫ��Ϊ__________ɫʱֹͣ�ζ�����ʱ����0.1 mol��L-1������V2 mL��
��4����V2��V1ʱ����ԭ������ɿ���Ϊ__________�����ţ���
| A��ֻ��NaOH |
| B��ֻ��Na2CO3 |
| C��Na2CO3��NaHCO3����� |
| D��NaOH��Na2CO3�Ļ���� |
��2���졡�ޡ����ҡ�ҡ������ƿ����Һ����ɫ�仯�͵ζ�����
��3���ơ���
��4��C
��3���ơ���
��4��C
Na2CO3�м������ᷴӦʱ����ʵ����H+��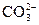 ��ϣ���������ļ����Ƚ��������HCO-3���ٽ������̼�Ტ�ֽ�����CO2��H2O��������ʵ�����ȼӷ�̪����pH����10ʱ�Էۺ�ɫ��pHΪ8��10ʱ��dz��ɫ����pHС��7ʱ��Ϊ��ɫ����ָʾ����ָʾ��ӦΪNaOH��HCl ="===" NaCl��H2O��Na2CO3��HCl ="===" NaCl��NaHCO3����Ӽ��ȣ���pHС��3.1ʱ�Ժ�ɫ��pHΪ3.1��4.4ʱ�ʳ�ɫ��pH����4.4ʱ�ʻ�ɫ����ָʾ����ָʾ��ӦΪ��NaHCO3��HCl ="===" NaCl��CO2����H2O������Ǵ�̼������Һ���������ᷴӦ�������ĵ������ɷ�Ӧ����ʽ��֪�����ʵ�����ȣ���V1=V2����V2��V1��������б�Ȼ����̼�����ơ�
��ϣ���������ļ����Ƚ��������HCO-3���ٽ������̼�Ტ�ֽ�����CO2��H2O��������ʵ�����ȼӷ�̪����pH����10ʱ�Էۺ�ɫ��pHΪ8��10ʱ��dz��ɫ����pHС��7ʱ��Ϊ��ɫ����ָʾ����ָʾ��ӦΪNaOH��HCl ="===" NaCl��H2O��Na2CO3��HCl ="===" NaCl��NaHCO3����Ӽ��ȣ���pHС��3.1ʱ�Ժ�ɫ��pHΪ3.1��4.4ʱ�ʳ�ɫ��pH����4.4ʱ�ʻ�ɫ����ָʾ����ָʾ��ӦΪ��NaHCO3��HCl ="===" NaCl��CO2����H2O������Ǵ�̼������Һ���������ᷴӦ�������ĵ������ɷ�Ӧ����ʽ��֪�����ʵ�����ȣ���V1=V2����V2��V1��������б�Ȼ����̼�����ơ�
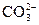 ��ϣ���������ļ����Ƚ��������HCO-3���ٽ������̼�Ტ�ֽ�����CO2��H2O��������ʵ�����ȼӷ�̪����pH����10ʱ�Էۺ�ɫ��pHΪ8��10ʱ��dz��ɫ����pHС��7ʱ��Ϊ��ɫ����ָʾ����ָʾ��ӦΪNaOH��HCl ="===" NaCl��H2O��Na2CO3��HCl ="===" NaCl��NaHCO3����Ӽ��ȣ���pHС��3.1ʱ�Ժ�ɫ��pHΪ3.1��4.4ʱ�ʳ�ɫ��pH����4.4ʱ�ʻ�ɫ����ָʾ����ָʾ��ӦΪ��NaHCO3��HCl ="===" NaCl��CO2����H2O������Ǵ�̼������Һ���������ᷴӦ�������ĵ������ɷ�Ӧ����ʽ��֪�����ʵ�����ȣ���V1=V2����V2��V1��������б�Ȼ����̼�����ơ�
��ϣ���������ļ����Ƚ��������HCO-3���ٽ������̼�Ტ�ֽ�����CO2��H2O��������ʵ�����ȼӷ�̪����pH����10ʱ�Էۺ�ɫ��pHΪ8��10ʱ��dz��ɫ����pHС��7ʱ��Ϊ��ɫ����ָʾ����ָʾ��ӦΪNaOH��HCl ="===" NaCl��H2O��Na2CO3��HCl ="===" NaCl��NaHCO3����Ӽ��ȣ���pHС��3.1ʱ�Ժ�ɫ��pHΪ3.1��4.4ʱ�ʳ�ɫ��pH����4.4ʱ�ʻ�ɫ����ָʾ����ָʾ��ӦΪ��NaHCO3��HCl ="===" NaCl��CO2����H2O������Ǵ�̼������Һ���������ᷴӦ�������ĵ������ɷ�Ӧ����ʽ��֪�����ʵ�����ȣ���V1=V2����V2��V1��������б�Ȼ����̼�����ơ�
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
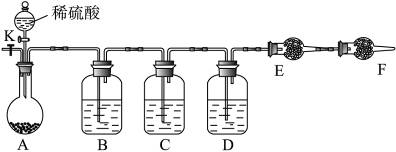
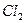 ʱ������NaOH��Һ����
ʱ������NaOH��Һ����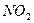 ʱ������ˮ����
ʱ������ˮ����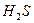 ʱ������Ũ
ʱ������Ũ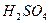 ����
����


 ���ڷ������ձ���
���ڷ������ձ���