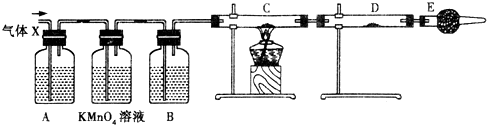
��Ŀ����
����ľ̿��ŨH2SO4��Ӧ�Ļ�ѧ����ʽ�� C+2H2SO4
| ||
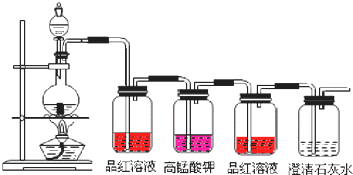
��֪ͼ��4��ϴ��ƿ��װ�м��Һ���ָ���4����Һ��Ʒ����Һ�����Ը��������Һ��Ʒ����Һ�ܳ���ʯ��ˮ��
�Իش��������⣮
��һ��ϴ��ƿ��Ʒ����Һ��ɫ�������˶����������
���������ݷ�Ӧ����ʽC+2H2SO4
CO2��+2SO2��+2H2O������Ļ��ϼ۱仯�ж�Ũ��������ã�Ʒ����ɫ��֤���˶����������Ư���ԣ��ݴ˿�֪�������ж����������ɣ���������ʹ���������Һ��Һ��ɫ��֤������������л�ԭ�ԣ��ݴ˽��н��
| ||
����⣺̼��Ũ����ķ�Ӧ�У�Ũ���������ǿ�����ԣ��ڷ�Ӧ��������������һ��ϴ��ƿ��Ʒ����Һ��ɫ�������˶����������Ư���ԣ��ݴ˿����ж�̼��Ũ����ķ�Ӧ���ж����������ɣ��ڶ���ϴ��ƿ�и��������Һ��ɫ�����������������Һ������֤���˶���������л�ԭ�ԣ�
�ʴ�Ϊ����������Ư�ף����鷴Ӧ�������ж����������ɣ���ԭ��
�ʴ�Ϊ����������Ư�ף����鷴Ӧ�������ж����������ɣ���ԭ��
���������⿼����Ũ��������ʣ���Ŀ�Ѷ��еȣ�ע������Ũ������еĻ�ѧ���ʣ��ܹ���ȷ��дŨ������̼��ͭ�ȷ�Ӧ�Ļ�ѧ����ʽ����ȷ���������������̼�������ķ������Ⱥ�˳��

��ϰ��ϵ�д�
�����Ŀ