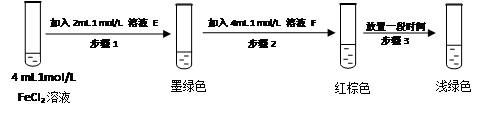��Ŀ����
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ�
��֪���������ԣ�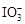 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ�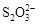 ��I������KI��I2
��I������KI��I2 KI3
KI3
��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ4�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
������ķ���Һ�м�K3Fe(CN)6��Һ�������Ƿ�õ�����������ɫ�ij������ɼ����Ƿ��� �������ӷ��ű�ʾ������ɫ�ij�����_______���û�ѧʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ��______��______
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧд����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O�������ʲ��ʺ���Ϊʳ�μӵ������������_________________________________��
��3��ijͬѧΪ̽���¶Ⱥͷ�Ӧ��Ũ�ȶԷ�Ӧ2IO3����5SO32����2H��=I2��5SO42����H2O�����ʵ�Ӱ�죬���ʵ�����±���ʾ��
�������ݣ�t1 t2���>������<����=����������V2=___________mL
��֪���������ԣ�
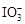 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ�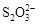 ��I������KI��I2
��I������KI��I2 KI3
KI3��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ4�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
������ķ���Һ�м�K3Fe(CN)6��Һ�������Ƿ�õ�����������ɫ�ij������ɼ����Ƿ��� �������ӷ��ű�ʾ������ɫ�ij�����_______���û�ѧʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ��______��______
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧд����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O�������ʲ��ʺ���Ϊʳ�μӵ������������_________________________________��
��3��ijͬѧΪ̽���¶Ⱥͷ�Ӧ��Ũ�ȶԷ�Ӧ2IO3����5SO32����2H��=I2��5SO42����H2O�����ʵ�Ӱ�죬���ʵ�����±���ʾ��
| | 0.01mol��L��1KIO3 ������Һ(������) �����/mL | 0.01mol��L��Na2SO3 ��Һ�����/mL | H2O�� ���/mL | ʵ�� �¶� /�� | ��Һ���� ��ɫʱ�� ��ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | t1 |
| ʵ��2 | 5 | 5 | 40 | 25 | t2 |
| ʵ��3 | 5 | 5 | V2 | 0 | t3 |
�������ݣ�t1 t2���>������<����=����������V2=___________mL
����14�֣�
��1���� Fe2�� �� Fe 3[Fe(CN)6]2 ����1�֣�
��IO3����5I����6H��=3I2��3H2O 2Fe3����2I��=2Fe2����I2 ����2�֣�
��2��O2��4KI��2H2O=2I2��4KOH ��2�֣�
KI3�����ȣ���ʪ�������²���I2��KI��KI������������I2����������2�֣�
��3��< �� 40 ����2�֣�
��1���� Fe2�� �� Fe 3[Fe(CN)6]2 ����1�֣�
��IO3����5I����6H��=3I2��3H2O 2Fe3����2I��=2Fe2����I2 ����2�֣�
��2��O2��4KI��2H2O=2I2��4KOH ��2�֣�
KI3�����ȣ���ʪ�������²���I2��KI��KI������������I2����������2�֣�
��3��< �� 40 ����2�֣�
���������
��1����Fe2�������軯����Һ��������Ӧ����
��Ӧ����Ϣ���������ԣ�IO3����Fe3����I2����˵��IO3����Fe3����������I������I2��
��2��KI����ʪ����������������ʧ�����Կ���I�������������γ�I2�����Ӷ������ʧ��
������֪��Ϣ����KI��I2
 KI3�����������ʲ��ȶ��ԣ������ֽ���̡�
KI3�����������ʲ��ȶ��ԣ������ֽ���̡���3���ɱ�����Ϣ��֪������ʵ��Ϊƽ�ж���ʵ�飬Ҫ��ѭ����һ�������Ĺ������������ʵ����Һ�������������50 ml�����V2="40" ml��ʵ��1��ʵ��2�Ƚϣ�ʵ��2��ˮ������࣬˵������Ũ�ȱ�ʵ��1�е�ҪС����Ӧ������������ʱ�䳤������t1��t2
���㶨λ�����������ӵļ��飬������ԭ��Ӧ����д��ʵ��������ݷ��������֪ʶ������ѧ�������Ϣ�Ķ�ȡ�ͷ�����������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 FeCl2+I2��
FeCl2+I2�� 3NaCl + NaClO4��NaClO4
3NaCl + NaClO4��NaClO4
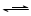 FeSO3��s��
FeSO3��s��