��Ŀ����
��16�֣�ijͬѧ��ͨ����ͼװ�ã��г�װ������ȥ��ʵ�飬̽��SO2��Na2O2��Ӧ�IJ��
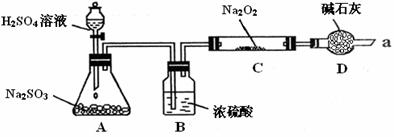
��װ��B������ ��
װ��D������ ��
����μ��鷴Ӧ���Ƿ���O2����
��
��C�й������������¼��裺
����1��ֻ��Na2SO3
����2��ֻ��Na2SO4
����3�� ��
��1������2�ķ�Ӧ����ʽΪ ��
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺

�ó����ۣ�������Na2SO4��
�÷����Ƿ���� ����ǡ����������� ��
��3�����ʵ����֤����3��ȡ����C�й���������Թ��У���������������ˮ�ܽ⣬ �������3������

��װ��B������ ��
װ��D������ ��
����μ��鷴Ӧ���Ƿ���O2����
��
��C�й������������¼��裺
����1��ֻ��Na2SO3
����2��ֻ��Na2SO4
����3�� ��
��1������2�ķ�Ӧ����ʽΪ ��
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺

�ó����ۣ�������Na2SO4��
�÷����Ƿ���� ����ǡ����������� ��
��3�����ʵ����֤����3��ȡ����C�й���������Թ��У���������������ˮ�ܽ⣬ �������3������
��16�֣�
��B������SO2���壬��ֹ�϶��ˮ������Na2O2��Ӧ����������2�֣�
D����ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ��ͬʱ���չ�����SO2��������Ⱦ����������������������������������������������������������2�֣�
���ô��û��ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ�ա�����������������2�֣�
����Na2SO3��Na2SO4 ������1�֣�
��1��Na2O2 + SO2 = Na2SO4 ������2�֣�
��2������1�֣�
HNO3�������ԣ��ݴ˲���ȷ��������Na2SO3����Na2SO4������С�����2�֣�
��3���ȼ��������ϡ���ᣬ�����ݼ�����ζ���壬�ټ���������BaCl2��Һ���ֲ�����ɫ�����������ȼ������BaCl2��Һ��������ɫ�������ټ��������ϡ���ᣬ��ɫ���������ܽ⣬�������ݼ�����ζ���塣���������Լ���ѡ���ʵ�������2�ֹ�4�֣�
��B������SO2���壬��ֹ�϶��ˮ������Na2O2��Ӧ����������2�֣�
D����ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ��ͬʱ���չ�����SO2��������Ⱦ����������������������������������������������������������2�֣�
���ô��û��ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ�ա�����������������2�֣�
����Na2SO3��Na2SO4 ������1�֣�
��1��Na2O2 + SO2 = Na2SO4 ������2�֣�
��2������1�֣�
HNO3�������ԣ��ݴ˲���ȷ��������Na2SO3����Na2SO4������С�����2�֣�
��3���ȼ��������ϡ���ᣬ�����ݼ�����ζ���壬�ټ���������BaCl2��Һ���ֲ�����ɫ�����������ȼ������BaCl2��Һ��������ɫ�������ټ��������ϡ���ᣬ��ɫ���������ܽ⣬�������ݼ�����ζ���塣���������Լ���ѡ���ʵ�������2�ֹ�4�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 �ԣ����۲쵽������ɫ�������ɴ˿�֪���ɵĺ��ƻ�����һ������ ��
�ԣ����۲쵽������ɫ�������ɴ˿�֪���ɵĺ��ƻ�����һ������ �� ��С����ˮ���Ĵ��ζ�����������˻˻����
��С����ˮ���Ĵ��ζ�����������˻˻���� 