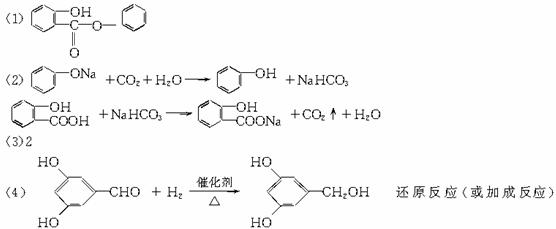
��Ŀ����
���ޣ�Salol����һ�������������ķ���ʽΪC13H10O3,�����ģ������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���
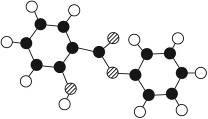
��1��������ͼģ��д�����Ľṹ��ʽ��___________��
��2������ˮ�⡢���롢�ᴿ�ɵõ������ı��Ӻ�ˮ���ᣨ���ǻ������ᣩ�������һ��������˵�����ӡ�̼�ᡢˮ���������������ǿ���û�ѧ����ʽ��ʾ��
__________________________________________________________________________
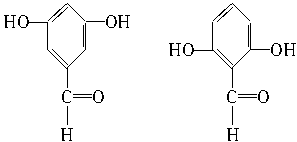
��3��ͬʱ��������Ҫ���ˮ�����ͬ���칹�干���֡�
�ٺ��б��������ܷ���������Ӧ������ϡNaOH��Һ�У�1mol��ͬ���칹������2molNaOH��Ӧ;��ֻ����������һ�ȴ��
��4���ӣ�3��ȷ����ͬ���칹������ѡһ�֣�ָ��Ϊ���п�ͼ�е�A��
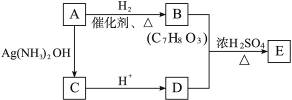
д������������Ӧ�Ļ�ѧ����ʽ��ˮ�����ýṹ��ʽ��ʾ������ָ����Ӧ�ķ�Ӧ���͡�
��A��B_____________________________________________________________________
��Ӧ����_________��
��B+D��E___________________________________________________________________
��Ӧ����_________��
��5������ˮ�����뱽�ӵĻ������ǵ����ʵ���֮��Ϊnmol���û������ȫȼ������O2aL��������bgH2O,cLCO2�����������Ϊ��״���µ������
�ٷֱ�д��ˮ����ͱ�����ȫȼ�յĻ�ѧ����ʽ���л�����÷���ʽ��ʾ��
__________________________________________________________________________
__________________________________________________________________________
����������ˮ��������ʵ���Ϊxmol���г�x�ļ���ʽ��
��1��C6H5��OOC��C6H4��OH
��2��
 +H2O+CO2��?
+H2O+CO2��?
(3)2
(4)�� �ӳɣ���ԭ����Ӧ
�ӳɣ���ԭ����Ӧ
��
 +H2O����������ȡ������Ӧ
+H2O����������ȡ������Ӧ
�� ���ӳɣ���ԭ����Ӧ
���ӳɣ���ԭ����Ӧ
 +H2O����������ȡ������Ӧ
+H2O����������ȡ������Ӧ
(5)��![]()
![]()
��x=c/22.4-6n����x=c/22.4-b/9
����:
��1�����ݽṹģ��ͼ����֪������ɫ����̼����ɫ�����⣬��ɫ������������ṹ��ʽΪ��
C6H5��OOC��C6H4��OH��
��2��CO2+C6H5ONa+H2O��C6H5OH+NaHCO3
2HO��C6H4��COOH+Na2CO3��2HO��C6H4��COONa+H2O+CO2��
��3��2�֣��ֱ��Ǽ�λ���λ��
��4��A��B��

�ӳɷ�Ӧ��
B+D��E��
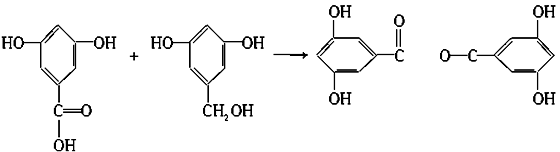 +H2O
+H2O
������Ӧ��ȡ����Ӧ����
��5��C7H6O3+7O2��7CO2+3H2O��C6H6O+7O2��6CO2+3H2O
x=c/22.4-6n(n=b/54,n=a/156.8)

 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д� ��
��



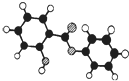 ��2013?����ģ�⣩���ޣ�Salol����һ����������Ϊ�����廯��������ģ������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���������˵������ȷ���ǣ�������
��2013?����ģ�⣩���ޣ�Salol����һ����������Ϊ�����廯��������ģ������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���������˵������ȷ���ǣ�������