��Ŀ����
��6�֣�X��Y��Z�������嶼�ܶԴ��������Ⱦ���ڹ�ҵ�϶������ü�Һ���ա���֪X�ǻ�ʯȼ��ȼ�յIJ���֮һ�����γ��������Ҫ���ʣ�Y��һ�ֵ��ʣ�����ˮ��Һ����Ư�����ã�Z�����Ṥҵ������β���е��к�����֮һ������ˮ��Ӧ����д�����з�Ӧ�ķ���ʽ��
��1��X��һ���������������ķ�Ӧ����ʽ ��
��2��Y������������Һ�����ӷ�Ӧ����ʽ ��
��3��Z��ˮ�ķ�Ӧ����ʽ ��
��1��2SO2+O2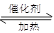 2SO3
2SO3
��2��Cl2+2OH-=ClO-+Cl-+H2O ��3��3NO2+H2O=NO+2HNO3
����

��ϰ��ϵ�д�
�����Ŀ