��Ŀ����
ij�о���ѧϰС��������ϵ�֪��Ư�������ᷴӦ�����Ƶ���������ѧ����ʽΪ��Ca(ClO)2+CaCl2+2H2SO4 2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣
2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣

�Իش�(1)��ʵ����A���ֵ�װ���ǣߣߣߣ�(��дװ�õ����)��

(2)�������һ��ʵ�飬֤��ϴ��ƿC�е�Na2SO3�Ѿ�������(����ʵ�鲽��)���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(3)��D�з�Ӧ�����Һ����Ư���ԣ���д��Dװ���з�����Ӧ�����ӷ���ʽ
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(4)��ʵ��������Ե�ȱ�ݣ���������Ľ��ķ����ߣߣߣߣߣߣߣߣߣߣߡ�
(5)��С���ֽ���������ʵ�飺��ȡƯ��2.0 g ����ĥ���ܽ⣬���Ƴ�250 mL��Һ��ȡ25 mL���뵽��ƿ�У��ټ��������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol/L��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ�� 2Na2S2O3+I2=Na2S4O6 + 2NaI ��Ӧ���ʱ������ȥNa2S2O3
20.0 mL�����Ư����Ca(ClO)2����������Ϊ���ߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(1)b (2)ȡC����Һ����������ϡ������Ȼ�����Һ�����Ƿ��������
(3)Cl2+HCO3-=CO2+Cl-+HClO
(4)����β������װ�� (5)35.75��
��������(1)���Ʊ�������ԭ���ǹ�Һ���ȣ�Ӧѡ��bװ�ã�(2)ϴ��ƿC�е�Na2SO3�����������ͻẬ����������ӣ�������������ӿɼ���ϡ������Ȼ�����Һ�����Ƿ����������(3)Cl2+HCO3-=CO2+Cl-+HClO��(4)�����ж�������ֱ���ŷŵ������У�ȱ��β������װ�á�(5)Ư�ۼ��������KI��Һ������H2SO4��Һ����ӦΪClO-+2I-+2H+=I2+Cl-+H2O,���2Na2S2O3+I2=Na2S4O6+ 2NaI�ɵã�Ca(ClO)2 ��4Na2S2O3 ��250 mL��Һ��Ca(ClO)2�����ʵ���Ϊ��0.02 L��0.1 mol/L��4��10=0.005 mol,Ư��2.0 g��Ca(ClO)2����������Ϊ35.75����

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д��������Ũ�������ܷ����ۻ���ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣ʵ�������������Լ���0.01 mol/L ����KMnO4��Һ��0.1 mol/L KI��Һ��3��H2O2��Һ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��.������Һ�еĽ������ӿ��ܺ���Fe2+��Fe3+�е�һ�ֻ����֣�
��.���������п��ܺ���_________�е�һ�ֻ����֡�
| ʵ����� | Ԥ������ | ���� |
��֤����� | ����٣�ȡ����0.01 mol/L����KMnO4��Һ������������Һ |
|
|
����ڣ�_________ |
| ����Fe3�� | |
��֤����� | ����������ͨ������װ�� |
| ������������ |
��ʵ��̽����
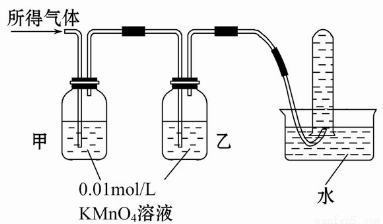
���������ۡ�
��ͬѧ�����������ѡ��KSCN��Һ���������KSCN��H2O2������Һ������ɲ���������̽�����жϸ÷����Ƿ���ȷ���������ۣ�_________��






