��Ŀ����
ijѧ�����ú���FeSO4�� Fe2��SO4�� 3��CuSO4��Һ�ᴿCuSO4�����ⶨͭ�����ԭ����������ʵ��������ͼ1��ʾ��

��֪��
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Cu2+ | 4.7 | 6.7 |
| ��ѡ����Լ��У�Cl2��H2O2��ŨH2SO4��NOH��CuO��Cu | ||
��1���Լ�A�Ļ�ѧʽΪ______�������Լ�A��Ӧ�����ӷ���ʽΪ______���Լ�B�Ļ�ѧʽΪ______������ B ��������______�������ٵ�������______��
��2������������������װ����ͼ2��ʾ���� X Ӧ��ֱ����Դ��______����Y�缫�Ϸ����ĵ缫��ӦʽΪ��______��
��3������ʵ�������Ҫ����______������ĸ����
A���������ǰ�缫������
B������缫�ں��ǰ������������ˮ��ϴ
C�����µ���缫�ϵ�ͭ������ϴ������
D���缫�ĺ�ɳ��صIJ����б��밴����ɡ����ء��ٺ�ɡ��ٳ��ؽ�������
E���ڿ����к�ɵ缫��������õ��º�ɷ�
��4����������Һ�м���ʯ̬��Һ���۲쵽��������______��
��5��ͭ�����ԭ�������ļ���ʽΪ______��
�⣺��1��FeSO4�� Fe2��SO4�� 3�Ļ��Һ�м���˫��ˮ������˫��ˮ���Խ�������������Ϊ���������ӣ���Ӧʵ���ǣ�2Fe2++2H++H2O2=2Fe3++2H2O���ټ�������ͭ���������ӳ�����pH�����Խ�����������������ͭ���Ӳ�������ʵ�ֳ�������Һ����ķ����ǹ��ˣ�
�ʴ�Ϊ��H2O2��2Fe2++2H++H2O2=2Fe3++2H2O��CuO��������Һ��pH��3.2-4.7֮�䣬ʹFe3+��ȫˮ��ΪFe��OH��3�����Ա��ȥ�����ˣ�
��2���������ͭ��Ҫ�������ϻ�ý���Cu�����������ϲ�������������ȷ������ת�Ƶ���������ȷ��ͭԪ�ص�ԭ���������Խ���ͭӦ��Ϊ�����������������������ӷ�����Ӧ������4OH--e-=2H2O+O2�����ʴ�Ϊ������4OH--4e-=2H2O+O2����
��3��A�������ϲ���ͭ�������Ǹ��ݵ��ǰ��缫�����ı仯���������ģ�������Ҫ�������ǰ�缫����������A��ȷ��
B������缫�ں��ǰ������������ˮ��ϴ�������������ϴȥ����B��ȷ��
C�������ϲ���ͭ�������Ǹ��ݵ��ǰ��缫�����ı仯���������ģ���C����
D���缫�ĺ�ɳ��صIJ����б��밴����ɡ����ء��ٺ�ɡ��ٳ��ؽ������εij����������Լ���ʵ������D��ȷ��
E���ڿ����к�ɵ缫��������õ��º�ɷ�������ᵼ�½���ͭ������֮��ķ�Ӧ����E��ȷ��
��ѡA��B��D��E
��4���������ͭ��Һ���������ᣬ��Һ��ʾ���ԣ���ʹ��ɫ��ʯ����Һ��ʾ��ɫ���ʴ�Ϊ����Һ��Ϊ��ɫ��
��5�����ݵ缫��Ӧʽ��������Cu2++2e-=Cu ������4OH--4e-=2H2O+O2����������VmL������ʱ�����ݷ�Ӧʽ����֪��ת�Ƶ���Ϊ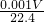 ��4mol=
��4mol=
mol������������Ӧ����Cu�����ԭ�������� =
= ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��������1�������Ի����£�˫��ˮ���Խ�������������Ϊ���������ӣ�����ͭ���Ժ��ᷢ����Ӧ�����κ�ˮ��ʵ�ֹ����Һ��ķ�����ù��˵ķ�����
��2�����ݵ��صĹ���ԭ��֪ʶ���ش�
��3������Cu���ԭ��������ȷ�������������ʵ�ʺͲ���֪ʶ���ش�
��4���������ͭ��Һ���������ᣬ��Һ��ʾ���ԣ��ݴ����ش�
��5�����������缫��Ӧ��ϵ����غ������㣮
���������⿼���������Ʊ�ʵ������жϣ����ʷ�����ᴿ�ķ����Լ����ԭ����Ӧ�ã�ʵ����̷������������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�
�ʴ�Ϊ��H2O2��2Fe2++2H++H2O2=2Fe3++2H2O��CuO��������Һ��pH��3.2-4.7֮�䣬ʹFe3+��ȫˮ��ΪFe��OH��3�����Ա��ȥ�����ˣ�
��2���������ͭ��Ҫ�������ϻ�ý���Cu�����������ϲ�������������ȷ������ת�Ƶ���������ȷ��ͭԪ�ص�ԭ���������Խ���ͭӦ��Ϊ�����������������������ӷ�����Ӧ������4OH--e-=2H2O+O2�����ʴ�Ϊ������4OH--4e-=2H2O+O2����
��3��A�������ϲ���ͭ�������Ǹ��ݵ��ǰ��缫�����ı仯���������ģ�������Ҫ�������ǰ�缫����������A��ȷ��
B������缫�ں��ǰ������������ˮ��ϴ�������������ϴȥ����B��ȷ��
C�������ϲ���ͭ�������Ǹ��ݵ��ǰ��缫�����ı仯���������ģ���C����
D���缫�ĺ�ɳ��صIJ����б��밴����ɡ����ء��ٺ�ɡ��ٳ��ؽ������εij����������Լ���ʵ������D��ȷ��
E���ڿ����к�ɵ缫��������õ��º�ɷ�������ᵼ�½���ͭ������֮��ķ�Ӧ����E��ȷ��
��ѡA��B��D��E
��4���������ͭ��Һ���������ᣬ��Һ��ʾ���ԣ���ʹ��ɫ��ʯ����Һ��ʾ��ɫ���ʴ�Ϊ����Һ��Ϊ��ɫ��
��5�����ݵ缫��Ӧʽ��������Cu2++2e-=Cu ������4OH--4e-=2H2O+O2����������VmL������ʱ�����ݷ�Ӧʽ����֪��ת�Ƶ���Ϊ
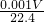 ��4mol=
��4mol=
mol������������Ӧ����Cu�����ԭ��������
 =
= ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����������1�������Ի����£�˫��ˮ���Խ�������������Ϊ���������ӣ�����ͭ���Ժ��ᷢ����Ӧ�����κ�ˮ��ʵ�ֹ����Һ��ķ�����ù��˵ķ�����
��2�����ݵ��صĹ���ԭ��֪ʶ���ش�
��3������Cu���ԭ��������ȷ�������������ʵ�ʺͲ���֪ʶ���ش�
��4���������ͭ��Һ���������ᣬ��Һ��ʾ���ԣ��ݴ����ش�
��5�����������缫��Ӧ��ϵ����غ������㣮
���������⿼���������Ʊ�ʵ������жϣ����ʷ�����ᴿ�ķ����Լ����ԭ����Ӧ�ã�ʵ����̷������������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
ijѧ�����ú���FeSO4�� Fe2��SO4�� 3��CuSO4��Һ�ᴿCuSO4�����ⶨͭ�����ԭ����������ʵ��������ͼ1��ʾ��

��֪��
�Իش��������⣺
��1���Լ�A�Ļ�ѧʽΪ______�������Լ�A��Ӧ�����ӷ���ʽΪ______���Լ�B�Ļ�ѧʽΪ______������ B ��������______�������ٵ�������______��
��2������������������װ����ͼ2��ʾ���� X Ӧ��ֱ����Դ��______����Y�缫�Ϸ����ĵ缫��ӦʽΪ��______��
��3������ʵ�������Ҫ����______������ĸ����
A���������ǰ�缫������
B������缫�ں��ǰ������������ˮ��ϴ
C�����µ���缫�ϵ�ͭ������ϴ������
D���缫�ĺ�ɳ��صIJ����б��밴����ɡ����ء��ٺ�ɡ��ٳ��ؽ�������
E���ڿ����к�ɵ缫��������õ��º�ɷ�
��4����������Һ�м���ʯ̬��Һ���۲쵽��������______��
��5��ͭ�����ԭ�������ļ���ʽΪ______��

��֪��
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Cu2+ | 4.7 | 6.7 |
| ��ѡ����Լ��У�Cl2��H2O2��ŨH2SO4��NOH��CuO��Cu | ||
��1���Լ�A�Ļ�ѧʽΪ______�������Լ�A��Ӧ�����ӷ���ʽΪ______���Լ�B�Ļ�ѧʽΪ______������ B ��������______�������ٵ�������______��
��2������������������װ����ͼ2��ʾ���� X Ӧ��ֱ����Դ��______����Y�缫�Ϸ����ĵ缫��ӦʽΪ��______��
��3������ʵ�������Ҫ����______������ĸ����
A���������ǰ�缫������
B������缫�ں��ǰ������������ˮ��ϴ
C�����µ���缫�ϵ�ͭ������ϴ������
D���缫�ĺ�ɳ��صIJ����б��밴����ɡ����ء��ٺ�ɡ��ٳ��ؽ�������
E���ڿ����к�ɵ缫��������õ��º�ɷ�
��4����������Һ�м���ʯ̬��Һ���۲쵽��������______��
��5��ͭ�����ԭ�������ļ���ʽΪ______��
