��Ŀ����
��12�֣�
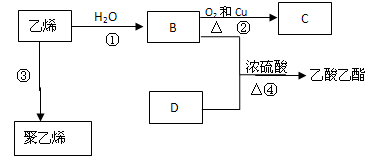
��1��������ʯ�����ڳ������Ƭ�������²�����ϩ�����������ʵ�ʵ�飬����������⡣

��A�����Ƭ�������� ��
��B�з�Ӧ������ ��C��ʵ�������� ����2����ȡ�����飨CH3CH2Cl������ѷ����ǣ��û�ѧ����ʽ��ʾ���� ����3���ÿ�������ˮ��SO2��Һ�еĻ�ѧ����ʽ�� ����4���Ѹɺ������� �����������ƣ������ա����պ�ĺ�������ˮ�У����˺����Һ���ữ��ӹ�����������ӷ���ʽ
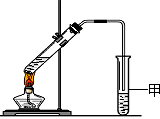
��5�����Թ�A�м���3 mL �Ҵ���Ȼ������Թܱ���������2 mL Ũ�����2 mL ���ᣬ����ͼ��ʾ���Ӻ�װ����ȡ����������
���Թ�B��ʢ�ű���Na2CO3��Һ�������ǣ��к����ᡢ�ܽ��Ҵ��� ��
��ʵ����ɺ���Ҫ����B�е�Һ�������Ҫ�õ��IJ���������Ҫ�� ��
��1��������ʯ�����ڳ������Ƭ�������²�����ϩ�����������ʵ�ʵ�飬����������⡣

��A�����Ƭ�������� ��
��B�з�Ӧ������ ��C��ʵ�������� ����2����ȡ�����飨CH3CH2Cl������ѷ����ǣ��û�ѧ����ʽ��ʾ���� ����3���ÿ�������ˮ��SO2��Һ�еĻ�ѧ����ʽ�� ����4���Ѹɺ������� �����������ƣ������ա����պ�ĺ�������ˮ�У����˺����Һ���ữ��ӹ�����������ӷ���ʽ
��5�����Թ�A�м���3 mL �Ҵ���Ȼ������Թܱ���������2 mL Ũ�����2 mL ���ᣬ����ͼ��ʾ���Ӻ�װ����ȡ����������
���Թ�B��ʢ�ű���Na2CO3��Һ�������ǣ��к����ᡢ�ܽ��Ҵ��� ��
��ʵ����ɺ���Ҫ����B�е�Һ�������Ҫ�õ��IJ���������Ҫ�� ��
��1���ٴ����ã���������Ӧ��������Ȼ�̼��ɫ��
��2��CH2===CH2�� HCl CH3CH2Cl��3��Br2+SO2+2H2O=H2SO4+2HBr
CH3CH2Cl��3��Br2+SO2+2H2O=H2SO4+2HBr
(4) ������H2O2+2I-+2H+=I2+2H2O
��5���ٽ��������������ܽ�� �ڷ�Һ©��
��2��CH2===CH2�� HCl
 CH3CH2Cl��3��Br2+SO2+2H2O=H2SO4+2HBr
CH3CH2Cl��3��Br2+SO2+2H2O=H2SO4+2HBr(4) ������H2O2+2I-+2H+=I2+2H2O
��5���ٽ��������������ܽ�� �ڷ�Һ©��
��1�������Ƭ�ܼӿ췴Ӧ���ʣ���������á�
����ϩ����̼̼˫���ܱ����Ը��������Һ������Ҳ�ܺ���ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ��
��2����ȡ�����飨CH3CH2Cl������ѷ�������ϩ���Ȼ���ļӳɷ�Ӧ������ʽΪCH2===CH2�� HCl CH3CH2Cl��
CH3CH2Cl��
��3����ˮ���������ԣ�������SO2���������ᣬ����ʽΪBr2+SO2+2H2O=H2SO4+2HBr��
��4�����������Ҫ��������˫��ˮ���������ԣ������������ӣ�����ʽΪH2O2+2I-+2H+=I2+2H2O��
��5���ٱ���̼������Һ���ܽ��ͽ��������������ܽ�ȡ�
����������������ˮ����Һ���ɣ���Ҫ���������Ƿ�Һ©����
����ϩ����̼̼˫���ܱ����Ը��������Һ������Ҳ�ܺ���ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ��
��2����ȡ�����飨CH3CH2Cl������ѷ�������ϩ���Ȼ���ļӳɷ�Ӧ������ʽΪCH2===CH2�� HCl
 CH3CH2Cl��
CH3CH2Cl����3����ˮ���������ԣ�������SO2���������ᣬ����ʽΪBr2+SO2+2H2O=H2SO4+2HBr��
��4�����������Ҫ��������˫��ˮ���������ԣ������������ӣ�����ʽΪH2O2+2I-+2H+=I2+2H2O��
��5���ٱ���̼������Һ���ܽ��ͽ��������������ܽ�ȡ�
����������������ˮ����Һ���ɣ���Ҫ���������Ƿ�Һ©����

��ϰ��ϵ�д�
�����Ŀ