��Ŀ����
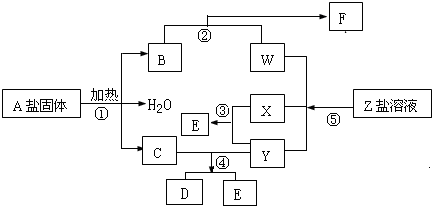
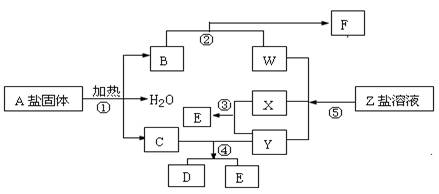
��15�֣���һ�������£�����ͬ�Ļ�ѧ��Ӧ����ʵ������ͼ�ĸ��ֱ仯������ֻ�з�Ӧ�١�������������ԭ��Ӧ��X��YΪ���ʣ�����Ϊ�����B��ֱ���ͷ��ӣ����ǻ�ѧ��ҵ�ϵ���Ҫ��Ӧ���ݴ�����գ�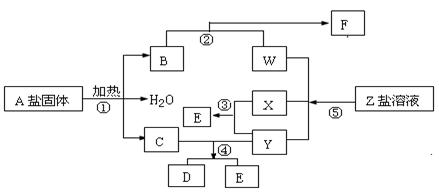
��1��д���������ʵĻ�ѧʽ��
A�� X�� Y�� ��
��2��д����Ӧ�ڵ����ӷ���ʽ�� ��
��3��1 g X��Y��ȫ��Ӧ����91.5 kJ����Ӧ�۵��Ȼ�ѧ����ʽ
��
��4����Ӧ����C��Y�����ʵ���֮��Ϊ1:3��D��ֻ������Ԫ�أ���D�� ��
��1��NH4HCO3��(NH4)2CO3 ��H2 Cl 2 ��ÿ��2�֣�
2 ��ÿ��2�֣�
��2��CO2+OH��= HCO3���� CO2+2OH��= CO32��+ H2O ��3�֣�
��3��H2(g) + Cl2(g) =" 2HCl" (g)����H= �� 183KJ/mol ��3�֣�
183KJ/mol ��3�֣�
��4��NCl3 ��3�֣�
����

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д� �żӾ���ϵ�д�
�żӾ���ϵ�д�
�����Ŀ