��Ŀ����
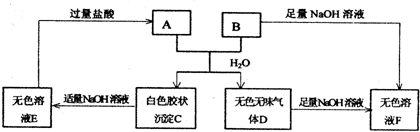
�ɶ�����Ԫ����ɵ���ѧ����������A��B��C��D��E�� X������ͼת����ϵ(����������ͷ�Ӧ������ȥ)�������ƶϲ���ȷ����(����)
A����X��Na2CO3��CΪCO2����Aһ������������D��E����Ӧ
B����A�ǵ��ʣ�B��D�ķ�Ӧ��OH����HCO3��==H2O��CO32������Eһ���ܻ�ԭFe2O3
C����DΪCO��C�ܺ�E��Ӧ����Aһ��ΪNa2O2�������ʽ�ǡ�![]()
D����DΪ��ɫ��������AĦ��������ȣ���Xһ��������

A

��ϰ��ϵ�д�
�����Ŀ
 �ס��ҡ����������ɶ�����Ԫ����ɵ����ʣ�����֮���������ת����ϵ��
�ס��ҡ����������ɶ�����Ԫ����ɵ����ʣ�����֮���������ת����ϵ��