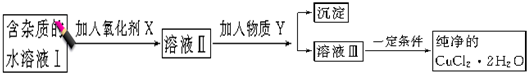
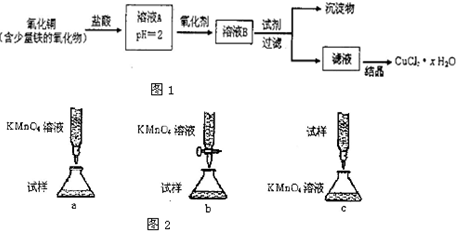
��Ŀ����
Ϊ�ⶨ��NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00 mL�����ȵ�120 �����ң�ʹ����ȫ���ݳ���(NH4)2SO4��NH4HSO4�ֽ��¶Ⱦ�����200 �桳������й�ʵ���������£���״������?
��1��ʵ������з������йط�Ӧ�����ӷ���ʽΪ��________��?
��2���ɢ�������ֱ���Ʋ⣺��״����3.7 g��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ________L��
��3���Լ���û�����еģ�NH4��2SO4��NH4HSO4���ʵ���֮��________��?
��4���������NaOH��Һ�����ʵ���Ũ��Ӧѡ���________�����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ________����(2)��(3)С��ֻ����������д��������̣���4��С�����д��������̡�
(1)H ++OH -�T�TH2O��NH![]() +OH -�T�TNH3��+H2O
+OH -�T�TNH3��+H2O
(2)3.7 gΪ������Ʒ����7.4 g��![]() ,�ʵõ����������Ϊ1.68 L��
,�ʵõ����������Ϊ1.68 L��![]() ��0.84 L��?
��0.84 L��?
��3�����������ݼ��㡣��n��(NH4)2SO4����n(NH4HSO4)�ֱ�Ϊx��y,��������?
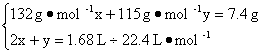
��ã�![]()
�ʣ�n��(NH4)2SO4����n(NH4HSO4)=0.012 5��0.05=1��4
��4��ӦѡNaOH����ȫ��Ӧ�ĵڢ��顣�ݣ�3�������ݣ�22.2 g��Ʒ�й�����n(H+)=n(NH4HSO4)=0.05 mol��![]() =0.15 mol;��H ++OH-�T�TH2O֪����NaOH 0.15 mol,��NH
=0.15 mol;��H ++OH-�T�TH2O֪����NaOH 0.15 mol,��NH![]() +OH -�T�TNH3��+H2O֪����1.12 L NH3����NaOH��
+OH -�T�TNH3��+H2O֪����1.12 L NH3����NaOH��![]() =0.05 mol,��NaOH��Һ�����ʵ���Ũ��Ϊ��
=0.05 mol,��NaOH��Һ�����ʵ���Ũ��Ϊ��
?c(NaOH)=![]() =5.0 mol��L -1??
=5.0 mol��L -1??
��������1��NH4HSO4���뷽��ʽΪNH4HSO4�T�TNH![]() +H ++SO
+H ++SO![]() ���ʼ���NaOH���������Ƿ���
���ʼ���NaOH���������Ƿ���
H ++OH -==H2O��Ȼ����NH![]() +OH -
+OH -![]() NH3��+H2O��?
NH3��+H2O��?
��2������������3.7 g��NaOH��������������Ϊԭ����һ�롣?
��3���������������ȱ��������߲���ʱ���ݼ��㣬��������Ʒ�������٣��ʸ��ݢ�����㡣
��4������NaOH���ʵ���Ũ��ʱ���밴NaOH����ʱ���ݼ��㣬�����ݿ���NaOH��㣬���ް������������ֻ����H ++OH -�T�TH2O�������жϷ�Ӧ�Ƿ���ȫ���ʱ���ѡ��������ݽ��м��㡣n(NH4HSO4)=0.05 mol��![]() =0.15 mol������n(NaOH)=0.15 mol+
=0.15 mol������n(NaOH)=0.15 mol+![]() =0.2 mol ,c(NaOH)=
=0.2 mol ,c(NaOH)=![]() =5.0 mol��L -1��
=5.0 mol��L -1��

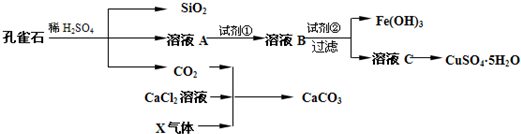 ��ش��������⣺
��ش��������⣺