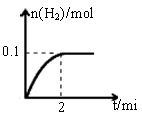��Ŀ����
(1)�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ����ÿС��2�֣���6�֣�
������������͵�������Ӧ
����ˮ�⣺
CH3CH(OH)CH3�Ĵ�������
(2)��4�֣�.��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2+2H2O+I2===H2SO4+2HI
��.2HI H2+I2 ��.2H2SO4===2SO2+O2+2H2O
H2+I2 ��.2H2SO4===2SO2+O2+2H2O
��1��һ���¶��£���1L�ܱ������м���1mol HI��g����������Ӧ�����ɵ�I2Ϊ���壬H2���ʵ�����ʱ��ı仯��ͼ��ʾ�� 0-2 min�ڵ�ƽ����Ӧ���ʦԣ�HI��=
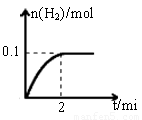
��2��ʵ������Zn��������ȡH2��Ϊ�˼ӿ췴Ӧ���ʣ����д�ʩ�����е��� ������ţ�
a������Ũ���� b����������CuSO4 ���� c���ô�п���洿п
d������ e.��п��Ū��п�� f.��98.3%Ũ����
(1) ��ÿС��3�֣���6�֣�
3CH3COOH��CH2OHCHOHCH2OH
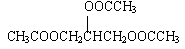 ��3H2O
��3H2O
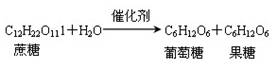
2CH3CHOHCH3��O2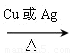 2CH3COCH3��2H2O
2CH3COCH3��2H2O
(2)�����֣���1��0.1mol/(L��min)---2��, ��2��a��f--2��
����������1�������к���3���ǻ������Ժ����ᷢ��������Ӧ������3�������ᡣ�����Ƕ��ǣ�ˮ�����������Ǻ��ǡ�2�������к��ǻ�������̼ԭ����ֻ��1����ԭ�ӣ��������IJ����DZ�ͪ��
��2������ͼ���֪��ƽ��ʱ���������ʵ�����0.1mol���������ĵ⻯����0.2mol������䷴Ӧ������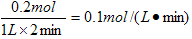 ��
��
��3��������������Է�Ӧ���ʵ�Ӱ�졣Ũ�����Ũ���ᶼ���������ᣬ��п��Ӧ�����ﲻ��������b��c�п��Թ���ԭ��أ���Ӧ���ʿ��Լӿ졣�¶����ߣ���Ӧ���ʼӿ졣e������Ӧ��ĽӴ��������Ӧ���ʼӿ졣���Դ�ѡaf��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� H2+I2 ��.2H2SO4===2SO2+O2+2H2O
H2+I2 ��.2H2SO4===2SO2+O2+2H2O