��Ŀ����
����ѡһ������ѧ�뼼����
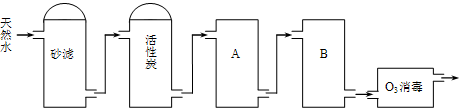
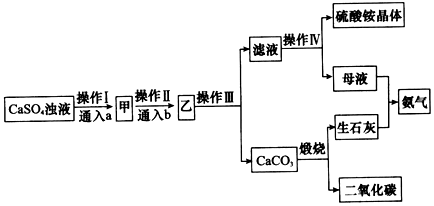
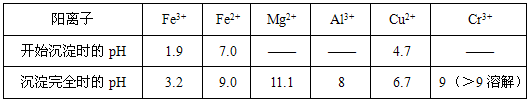
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ������ͼ��ʾ��
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ������ͼ��ʾ��
(1)��ȥ�����е�Ca2+��Mg2+��SO42-���ӣ��������г�������˳����________������ţ���
a.Na2CO3 b.NaOH c.BaCl2
(2)����Һ��pH�������Գ�ȥ��������____________��
(3)����Ȼ���ϡ��Һ���Ʊ���84����Һ����ͨ��ʱ��������Һ��ȫ���գ�����������Һ����һ�����ʣ�д����Ӧ�Ļ�ѧ����ʽ��________��
(4)��������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ��_________________��
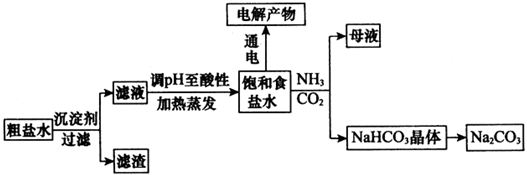
(5)ij̽���С�齫������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ����ͼ��ʾ��ͼ�мг֡��̶��õ�����δ��������
a.Na2CO3 b.NaOH c.BaCl2
(2)����Һ��pH�������Գ�ȥ��������____________��
(3)����Ȼ���ϡ��Һ���Ʊ���84����Һ����ͨ��ʱ��������Һ��ȫ���գ�����������Һ����һ�����ʣ�д����Ӧ�Ļ�ѧ����ʽ��________��
(4)��������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ��_________________��
(5)ij̽���С�齫������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ����ͼ��ʾ��ͼ�мг֡��̶��õ�����δ��������

�Իش������й����⣺
����װ���е��Լ���________��
�ڶ�װ����ϡ�����������________��
��ʵ����������NaHCO3����IJ�����____���������������ƣ���
(6)�����������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۣ���ԭ����_______________��������ӷ���ʽ��������
�ڹ�ҵ�ϣ������ô�������ռ�����ijЩ������Ʒ�����ñ��ʹ�����Һ��Cl2��Ӧ��ȡ��Ч�ɷ�ΪNaClO������Һ���䷴Ӧ�����ӷ���ʽ��______������֪̼�������ǿ�ڴ����ᣩ
����װ���е��Լ���________��
�ڶ�װ����ϡ�����������________��
��ʵ����������NaHCO3����IJ�����____���������������ƣ���
(6)�����������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۣ���ԭ����_______________��������ӷ���ʽ��������
�ڹ�ҵ�ϣ������ô�������ռ�����ijЩ������Ʒ�����ñ��ʹ�����Һ��Cl2��Ӧ��ȡ��Ч�ɷ�ΪNaClO������Һ���䷴Ӧ�����ӷ���ʽ��______������֪̼�������ǿ�ڴ����ᣩ
(1)cab(��cba��bca)
(2)CO32-��OH-
(3)NaCl+H2O NaClO+H2��
NaClO+H2��
(4)NH3
(5)�ٱ���̼��������Һ��������δ��Ӧ��NH3���۹���
(6)��CO32-ˮ���Լ��ԣ�CO32-+H2O HCO3-+OH-�������ڼ���������ˮ�⣬�ܴﵽȥ��Ŀ�ģ���2CO32-+Cl2+H2O= Cl-+ClO-+2HCO3-
HCO3-+OH-�������ڼ���������ˮ�⣬�ܴﵽȥ��Ŀ�ģ���2CO32-+Cl2+H2O= Cl-+ClO-+2HCO3-
(2)CO32-��OH-
(3)NaCl+H2O
 NaClO+H2��
NaClO+H2�� (4)NH3
(5)�ٱ���̼��������Һ��������δ��Ӧ��NH3���۹���
(6)��CO32-ˮ���Լ��ԣ�CO32-+H2O
 HCO3-+OH-�������ڼ���������ˮ�⣬�ܴﵽȥ��Ŀ�ģ���2CO32-+Cl2+H2O= Cl-+ClO-+2HCO3-
HCO3-+OH-�������ڼ���������ˮ�⣬�ܴﵽȥ��Ŀ�ģ���2CO32-+Cl2+H2O= Cl-+ClO-+2HCO3- 
��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ
 CH3CH2Cl��HCl
CH3CH2Cl��HCl  CH3CH2Cl
CH3CH2Cl