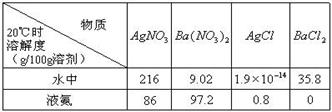
��Ŀ����
��1���ü���������һ�����۵����ⷽ�����йص��Ȼ�ѧ����ʽ����:
CH4(g)+1/2O2(g) CO(g)+2H2(g)����H= ��36kJ/mol������ ��
CO(g)+2H2(g)����H= ��36kJ/mol������ ��
CH4(g)+H2O(g) CO(g)+3H2(g)����H= +216kJ/mol����������
CO(g)+3H2(g)����H= +216kJ/mol����������
������˵����ȷ���� ____
������ʵ���֮��Ϊ6��1
��ij�¶��£���100L��Ӧ���г�������ˮ���������ʵ����ֱ�Ϊ100mol��300molʱ���ٶ�ֻ������ӦCH4(g)+H2O(g) CO(g)+3H2(g)���������ת����Ϊ0.5ʱ����ʱƽ�ⳣ��Ϊ _______
CO(g)+3H2(g)���������ת����Ϊ0.5ʱ����ʱƽ�ⳣ��Ϊ _______
��2������Һ����NaNO3��NaNO2��NaOH��ɵģ��ڼ��������£�ͨ��������Ӧʹ����������һ�����ܵ�Fe3O4��
3Fe+NaNO2+5NaOH 3Na2FeO2+H2O+NH3�����������٣�
3Na2FeO2+H2O+NH3�����������٣�
8Fe+3NaNO3+5NaOH+2H2O 4Na2Fe2O4+3NH3�������ڣ�
4Na2Fe2O4+3NH3�������ڣ�
�Լ������������������������벹���������Ӧ�ķ���ʽ����ƽ�� __________________
CH4(g)+1/2O2(g)
 CO(g)+2H2(g)����H= ��36kJ/mol������ ��
CO(g)+2H2(g)����H= ��36kJ/mol������ ��CH4(g)+H2O(g)
 CO(g)+3H2(g)����H= +216kJ/mol����������
CO(g)+3H2(g)����H= +216kJ/mol����������������˵����ȷ���� ____
| A��2H2O(l)=2H2(g)+O2(g) ��H=+504kJ/mol |
| B������Ӧ���Ц�(CH4)��=��(CO)��ʱ�������÷�Ӧ��ƽ��״̬ |
| C��������������ʱ��������ϵѹǿ����Ӧ�٢��м����ת���ʾ����� |
| D��Ϊά�ֺ㶨�¶ȣ�������������ʧ����ij��Ӧ����ͬʱ������Ӧ�����ʱ�����ļ� |
��ij�¶��£���100L��Ӧ���г�������ˮ���������ʵ����ֱ�Ϊ100mol��300molʱ���ٶ�ֻ������ӦCH4(g)+H2O(g)
 CO(g)+3H2(g)���������ת����Ϊ0.5ʱ����ʱƽ�ⳣ��Ϊ _______
CO(g)+3H2(g)���������ת����Ϊ0.5ʱ����ʱƽ�ⳣ��Ϊ _______��2������Һ����NaNO3��NaNO2��NaOH��ɵģ��ڼ��������£�ͨ��������Ӧʹ����������һ�����ܵ�Fe3O4��
3Fe+NaNO2+5NaOH
 3Na2FeO2+H2O+NH3�����������٣�
3Na2FeO2+H2O+NH3�����������٣�8Fe+3NaNO3+5NaOH+2H2O
 4Na2Fe2O4+3NH3�������ڣ�
4Na2Fe2O4+3NH3�������ڣ��Լ������������������������벹���������Ӧ�ķ���ʽ����ƽ�� __________________
��1����D ��1.35 ��2��Na2FeO2+Na2Fe2O4+2H2O=Fe3O4��+4NaOH
�����������1����A.(�� -��)��2�ɵ�2H2O(g)
 O2(g)+2H2(g) ��H= +504kJ/mol.����.B. ���ڷ�Ӧ���ں�ʱ�̶��Ц�(CH4)��=��(CO)�����ʲ��ܱ����÷�Ӧ��ƽ��״̬������C����Ӧ�٢ڵ�����Ӧ���������������ķ�Ӧ�����ԣ�������������ʱ��������ϵѹǿ����Ӧ�٢ڵ�ƽ�ⶼ�����淴Ӧ�����ƶ����ʶ����еļ����ת���ʾ���С������D�����跢���ٵļ�������ʵ���ΪX�������ڵļ�������ʵ���ΪY��Ϊά�ֺ㶨�¶ȣ�������������ʧ����ij��Ӧ����ͬʱ������Ӧ�����ʱ����36X=216Y�����X��Y="6:1." ���ļ�������ʵ���֮��Ϊ6��1����ȷ��ѡ��Ϊ��D���ڻ�ѧƽ�ⳣ��K={C(CO)��C3(H2)}/{C(CH4)��C(H2O)}={0.5��1.53}{0.5��2.5}=1.35.��2����������Ӧ�ķ���ʽΪ��Na2FeO2+Na2Fe2O4+2H2O=Fe3O4��+4NaOH
O2(g)+2H2(g) ��H= +504kJ/mol.����.B. ���ڷ�Ӧ���ں�ʱ�̶��Ц�(CH4)��=��(CO)�����ʲ��ܱ����÷�Ӧ��ƽ��״̬������C����Ӧ�٢ڵ�����Ӧ���������������ķ�Ӧ�����ԣ�������������ʱ��������ϵѹǿ����Ӧ�٢ڵ�ƽ�ⶼ�����淴Ӧ�����ƶ����ʶ����еļ����ת���ʾ���С������D�����跢���ٵļ�������ʵ���ΪX�������ڵļ�������ʵ���ΪY��Ϊά�ֺ㶨�¶ȣ�������������ʧ����ij��Ӧ����ͬʱ������Ӧ�����ʱ����36X=216Y�����X��Y="6:1." ���ļ�������ʵ���֮��Ϊ6��1����ȷ��ѡ��Ϊ��D���ڻ�ѧƽ�ⳣ��K={C(CO)��C3(H2)}/{C(CH4)��C(H2O)}={0.5��1.53}{0.5��2.5}=1.35.��2����������Ӧ�ķ���ʽΪ��Na2FeO2+Na2Fe2O4+2H2O=Fe3O4��+4NaOH
��ϰ��ϵ�д�
�����Ŀ
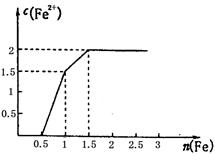
 mol��L-1
mol��L-1 mol��L-1
mol��L-1 mol��L-1
mol��L-1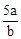 mol��L-1
mol��L-1