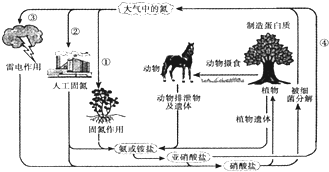
��Ŀ����
�Ժϳɰ�����������������ͼ���Իش�������⣺

(1)��ͬ��ԭ�����������侭��ָ�����±���
���ϱ����ݼ���Ȼ��Դ������߷�չǰ������________��
A����Ȼ�� B��ʯ���� C������ D��ú��
(2)������һ����ͨ��������������ʵ�ֵģ�ͨ��������Ӧ��������ɽ�����������õ��������벹���������Ӧ��
��MezOy��zH2S��(y��z)H2=MezSz��yH2O
��MezSz��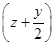 O2=MezOy��zSO2
O2=MezOy��zSO2
��__________________________________________________
(3)����ľ��ơ�������������������������CO���к����塣���õķ�����ͭϴ���ͼ��黯����CO(g)��Cu(NH3)2Ac��NH3(l)??[Cu(NH3)3CO]Ac������Ӧ���ȣ���Ӧ��ͭϴ���н��У����պ��ͭҺ�͵��������У���������������CO������������________��������ͭҺѭ��ʹ�á����黯�����ǰ�CO��CO2ת��Ϊ���ϳ�����CH4����Ҫ��Ӧ��CO(g)��3H2(g) CH4(g)��H2O(g)������Ӧ���ȣ����黯��������__________________��
CH4(g)��H2O(g)������Ӧ���ȣ����黯��������__________________��
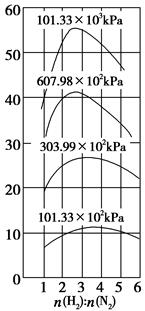
(4)500 ��ʱ��y��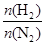 ��ѹǿ�ı仯��ϵ�������������____________�����Ŀǰ������������Ӧ���Ƶ�
��ѹǿ�ı仯��ϵ�������������____________�����Ŀǰ������������Ӧ���Ƶ�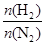 ��________��
��________��

(1)��ͬ��ԭ�����������侭��ָ�����±���
| | ��Ȼ�� | ʯ���� | ���� | ú�� |
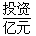 | 5.6 | 6.5 | 8.0 | �� |
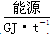 | 28��30 | 35.5 | 41.8 | 54.4 |
 | 257 | 390��447 | 220��280 | 500 |
���ϱ����ݼ���Ȼ��Դ������߷�չǰ������________��
A����Ȼ�� B��ʯ���� C������ D��ú��
(2)������һ����ͨ��������������ʵ�ֵģ�ͨ��������Ӧ��������ɽ�����������õ��������벹���������Ӧ��
��MezOy��zH2S��(y��z)H2=MezSz��yH2O
��MezSz��
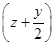 O2=MezOy��zSO2
O2=MezOy��zSO2��__________________________________________________
(3)����ľ��ơ�������������������������CO���к����塣���õķ�����ͭϴ���ͼ��黯����CO(g)��Cu(NH3)2Ac��NH3(l)??[Cu(NH3)3CO]Ac������Ӧ���ȣ���Ӧ��ͭϴ���н��У����պ��ͭҺ�͵��������У���������������CO������������________��������ͭҺѭ��ʹ�á����黯�����ǰ�CO��CO2ת��Ϊ���ϳ�����CH4����Ҫ��Ӧ��CO(g)��3H2(g)
 CH4(g)��H2O(g)������Ӧ���ȣ����黯��������__________________��
CH4(g)��H2O(g)������Ӧ���ȣ����黯��������__________________��
(4)500 ��ʱ��y��
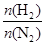 ��ѹǿ�ı仯��ϵ�������������____________�����Ŀǰ������������Ӧ���Ƶ�
��ѹǿ�ı仯��ϵ�������������____________�����Ŀǰ������������Ӧ���Ƶ�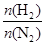 ��________��
��________��(1)A
(2)MezSz��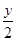 SO2=MezOy��
SO2=MezOy��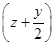 S
S
(3)��ѹ�ͼ��ȡ���ѹ���ʵ��¶�
(4)ƽ��ʱ��������а����������������С��3��1
(2)MezSz��
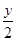 SO2=MezOy��
SO2=MezOy��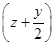 S
S(3)��ѹ�ͼ��ȡ���ѹ���ʵ��¶�
(4)ƽ��ʱ��������а����������������С��3��1
���⿼����ƽ��ԭ���ڹ�ҵ�����е�Ӧ�á�(1)��չǰ��һ����ӳɱ��Ƚϣ���һ���滹Ҫ�Ƚ��������ģ��ۺ�������Ȼ������ǰ����(2)���䷽��ʽ�Ĺؼ����������⣬������ɽ�������������ʡ���Ӧ��������SO2��Ҫ��SO2ת�������ʡ��������������ԣ�2�ۣ�SO2�����ԣ�4�ۣ�ͨ�����߹��з�Ӧ���Ƶ���(3)��CO����������Ҫʹƽ�����淴Ӧ�����ƶ������黯��������ƽ�������ƶ�����ѹ�������¶ȡ�(4)ѹǿ����ƽ��������Ӧ�����ƶ����۲�ͼ��yҲ�������H2�ı�����y��������С��y������ƽ��ʱ��������а���������������۲�ͼ���֪����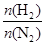 ��С��3��1ʱ�������������������ͬ���������
��С��3��1ʱ�������������������ͬ���������
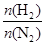 ��С��3��1ʱ�������������������ͬ���������
��С��3��1ʱ�������������������ͬ���������
��ϰ��ϵ�д�
�����Ŀ
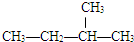 ��
��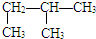 ��Ϊͬ���칹��
��Ϊͬ���칹��