��Ŀ����
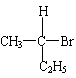
��10�֣����й����ű������ҹ���2000��11��16���𣬽�ֹʹ�ú�PPA�Ŀ���ðҩ�PPA�DZ���������Ӣ����д����ṹ��ʽΪ��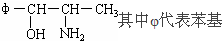
PPA�ܷ�������������ƽ������Ҫ�����˷��ð������֧�����ȡ���PPA��ʹ�������˷ܣ����²�����ʧ�ߡ����ù���ʱ������Ѫѹ���з�ȡ���ش��������⣺
��1��PPA����ǡ����ǡ��������������ķ���ʽΪ�� ��
��2��PPA���ܾ��е����ʺͿ��ܷ����ķ�Ӧ�� ������ţ���
��������Ӧ ����ȥ��Ӧ �۾������� ����Br2ȡ�� �ݼӾ�
��3��һ��̼ԭ�����ĸ���ͬԭ�ӻ��������ʱ���ͳƴ�̼ԭ��Ϊ����̼ԭ�ӣ�����ͼ��������������̼ԭ�ӡ���PPA�����ں���
������̼ԭ�ӡ�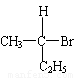
��4���л���A��B��PPA�ķ���ʽ��ͬ��A��һ�������£��ɱ�����Ϊ�������ᡣ��A�Ľṹ��ʽΪ ��
B����FeCl3��Һ����ɫ��Ӧ����д��B��һ�ֽṹ��ʽ ��
��5����֪����������NaNO2�ڴ��������·�Ӧ���ɴ����磺
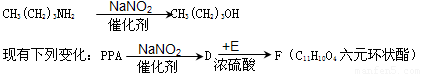
��E���ʵ�����Ϊ ��F�ĽṹʽΪ ��
д��D��E��Ӧ������Ԫ��״��������Ļ�ѧ����ʽ��
��
��1������ C9H13NO ��2���٢ڢ� ��3�� 2
��4��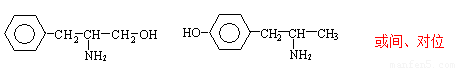

��5���Ҷ���
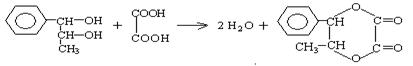
����������1�����б�����̼�⻯�����Ƿ��������÷��Ӻ��е�����ԭ�ӣ����Բ��Ƿ������������ʽΪC9H13NO��
��2�����ݽṹ��ʽ��֪�������к��а������ǻ������Ԣ٢ڢܶ���ȷ���û�����û�����ԣ�Ҳ���ܷ����Ӿ۷�Ӧ��
��3����������̼ԭ�ӵĸ����֪�����ǻ�������̼ԭ���Լ��Ͱ���������̼ԭ�Ӷ�������̼ԭ�ӡ�
��4��A��һ�������£��ɱ�����Ϊ�������ᣬ˵��A�е��ǻ��ܱ����������Ȼ�������A�Ľṹ��ʽΪ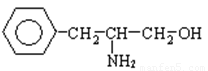 ��B����FeCl3��Һ����ɫ��Ӧ��˵�����з��ǻ�������ܵĽṹ��ʽΪ
��B����FeCl3��Һ����ɫ��Ӧ��˵�����з��ǻ�������ܵĽṹ��ʽΪ ����λ���λҲ���ԣ���
����λ���λҲ���ԣ���
��5��������֪��Ϣ��֪��D�Ľṹ��ʽΪ ������D��E��Ӧ����F��������F�ķ���ʽ��֪���÷�Ӧ��������Ӧ�����Ը���ԭ���غ��֪��E���Ҷ��ᣬ��Ӧ�ķ���ʽΪ
������D��E��Ӧ����F��������F�ķ���ʽ��֪���÷�Ӧ��������Ӧ�����Ը���ԭ���غ��֪��E���Ҷ��ᣬ��Ӧ�ķ���ʽΪ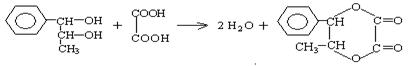 ��
��

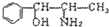 ������ʽ���л���ṹ����һ��ʾ������PPA�ļ���ʽ��
������ʽ���л���ṹ����һ��ʾ������PPA�ļ���ʽ��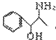 ��PPA�ܷ�������������ֹ�ȣ���Ҫ�����˷��ð��������֧�����ȣ���PPA��ʹ�������˷ܣ����²�����ʧ�ߣ����ù���ʱ������Ѫѹ���з�ȣ���ش��������⣺
��PPA�ܷ�������������ֹ�ȣ���Ҫ�����˷��ð��������֧�����ȣ���PPA��ʹ�������˷ܣ����²�����ʧ�ߣ����ù���ʱ������Ѫѹ���з�ȣ���ش��������⣺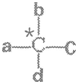
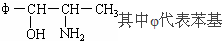
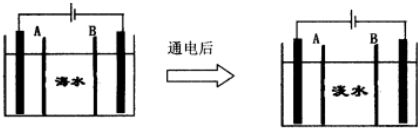
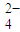 �����ӣ��缫Ϊ���Ե缫��������������⣺
�����ӣ��缫Ϊ���Ե缫��������������⣺