��Ŀ����
��11�֣���ͼ�ֱ�ΪNaCl��CsCl�����ʯ�ľ���ṹģ�͡�
�� ��NaCl�����У���ÿ��Na+���ӵ���Χ������ӽ����Ҿ�����ȵ�Na+���ӹ���_____������һ��NaCl�����У�Cl-���ӵĸ�������________��Na+���ӵĸ�������________��
�� ��CsCl�����У�ÿ��Cs+������Χ������ӽ����Ҿ�����ȵ�Cs+���ӹ���_____����ÿ��Cs+������Χ������ӽ����Ҿ�����ȵ�Cl-���ӹ���_____����
�� �ڽ��ʯ�����У�ÿ��Cԭ����Χ��________��C��C����1 mol���ʯ���� C��C��________mol��
�� ��������ʯ����ṹģ���е�Cԭ�ӻ���Siԭ�ӣ�����ÿ��Si��Si���м����Oԭ�Ӿͳ���SiO2�ľ���ṹģ�͡���ô��SiO2�����У�ÿ��Siԭ����Χ��______��Oԭ�ӣ�ÿ��Oԭ��_______��Siԭ�ӣ�ÿ��Siԭ����Χ��_______��Si��O����1mol SiO2�к���_____mol Si��O����
��1��12 4 4 ��2��6 8 ��3��4 2 ��4��4 2 4 4
����:��

��ϰ��ϵ�д�
�����Ŀ



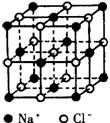


 �� ��NaCl�����У���ÿ��Na+���ӵ���Χ������ӽ����Ҿ�����ȵ�Na+���ӹ���_____������һ��NaCl�����У�Cl-���ӵĸ�������________��Na+���ӵĸ�������________��
�� ��NaCl�����У���ÿ��Na+���ӵ���Χ������ӽ����Ҿ�����ȵ�Na+���ӹ���_____������һ��NaCl�����У�Cl-���ӵĸ�������________��Na+���ӵĸ�������________��