��Ŀ����
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A��������ϡ���ᣬ��Һ��Ϊdz��ɫ��Fe+4H��+NO3��=Fe3++NO��ʮ2H2O |
| B����KIO3����������Һ�е�KI: 5I�� +IO3��+3H2O =3I2+6OH�� |
C����ˮ���ᣨ 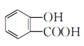 ���еμ�NaHCO3��Һ���ų���ɫ���壺 ���еμ�NaHCO3��Һ���ų���ɫ���壺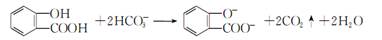 |
| D��0.01mol��L��l NH4 Al(SO4)2��Һ��0.02 mol��L��l Ba(OH)2��Һ�������ϣ�NH4��+Al3��+2SO42��+2Ba2��+4OH��=2BaSO4 ��ʮAl(OH)3��+NH3��H2O |
D
�������������������ϡ���ᣬ��Һ��Ϊdz��ɫ��˵�����ɵ���Fe2��,������Fe3��,��Aѡ�������KIO3����������Һ�е�KI��������Һ�в���������OH����Bѡ�������ˮ�����еμ�NaHCO3��Һ��ˮ�����з��ǻ�����HCO3����Ӧ��Cѡ�����Dѡ����ȷ��
���㣺���������ӷ���ʽ��

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д��������ӷ�Ӧ����ʽ��ȷ����( )
| A����ˮ���չ�����SO2��OH����SO2��HSO3�� |
| B��FeSO4��Һ������������4Fe2����O2��2H2O��4Fe3����4OH�� |
| C��NaAlO2��Һ�м������ϡ���AlO2����H����H2O��Al(OH)3�� |
| D��Cl2��ϡNaOH��Һ��Ӧ��Cl2+2OH����Cl��+ClO��+ H2O |
��ij��ɫ��Һ�л����ص���NaOH��Һֱ����������������������������NaOH��Һ����Ĺ�ϵ����ͼ��ʾ���ɴ�ȷ����ԭ��Һ�к��е��������ǣ�( )
| A��H+��Mg2+��Al3+ | B��Mg2+��Al3+��Fe2+ |
| C��H+��Ba2+��Al3+ | D��ֻ��Mg2+��Al3+ |
ij��Һ�к�����NH4+��Na+��HCO3����CO32����CH3COO�� ���ӣ����м���������Na2O2�������Һ������Ũ�Ȼ������ֲ�����ǣ�������Һ����ޱ仯�� �� ��
| A��Na+ | B��CH3COO�� | C��CO32����NH4+ | D��CH3COO����Na+ |
ij������Һ�п��ܺ���NO2����SO42����SO32����CO32����Cl����NO3���������ӡ�ijͬѧȡ5�ݴ���Һ��Ʒ���ֱ����������ʵ�飺
����pH�Ʋ����ҺpH����7
�ڼ������ᣬ������ɫ�̼�������
�ۼ��������ữ��AgNO3��Һ������ɫ�������ҷų���ɫ�̼�������
�ܼ�����BaCl2��Һ��������ɫ�������ó�����ȫ����ϡ�����ҷų����壬������ͨ��Ʒ����Һ����Һ����ɫ
�ݼ�����BaCl2��Һ��������ɫ����������Һ�м����ữ�ģ�NH4��2Fe��SO4��2��Һ���ٵμ�KSCN��Һ���Ժ�ɫ
������˵������ȷ����
| A���ɢ��е�ʵ��������Ʋ�һ������NO2�� |
| B�����ݢڢۢܿ�ȷ��һ������NO2����CO32����Cl������������ |
| C������ȷ���Ƿ�һ������NO3�� |
| D���ɢܼ���ȷ��һ��������SO42����SO32�� |
������ط�Ӧ�����ӷ���ʽ��д��ȷ���ǣ� ��
| A������������������Fe(OH)3��3H��=Fe3����3H2O |
| B������ͭ��Һ�����ԣ�Cu2����2H2O=Cu(OH)2����2H�� |
| C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ�NH4+��OH��=NH3����H2O |
| D�����ữ�ĸ��������Һ����˫��ˮ��2MnO4-��6H����5H2O2=2Mn2����5O2����8H2O |
���и�������,һ������ָ�������д����������(����)
| A���ں��д���I-����Һ��:Cl-��Fe3+��Al3+��Cu2+ |
| B������ˮ�������c(H+)=10-12 mol��L-1����Һ��:Na+��Ba2+��Cl-��Br- |
| C����ʹpH��ֽ������Һ��:Fe2+��Na+��SO42����ClO- |
| D���ڼ���Al�ܷų�����H2����Һ��:NH4+��SO42����Cl-��HCO3�� |
�ձ���ʢ��100 mL 1 mol/L��NaHSO4��Һ,��������εμ�1 mol/L��Ba(OH)2��Һ,�ձ���ijЩ����(����)�����ʵ����ı仯������ͼ������˵������ȷ����(����)
| A������a��ʾBa2+�����ʵ����ı仯 |
| B������c��ʾOH-�����ʵ����ı仯 |
C������Ba(OH)2��Һ50 mL��Ӧ�����ӷ���ʽΪBa2++OH-+H++SO42�� BaSO4��+H2O BaSO4��+H2O |
D������Ba(OH)2��Һ����50 mL��,��Ӧ�����ӷ���ʽΪOH-+H+ H2O H2O |
���н�����������ʵ������ӷ���ʽ����ȷ����(����)
A��NaClO��Һ�ʼ��ԣ�ClO����H2O HClO��OH�� HClO��OH�� |
B�����ˮ�еμӱ���FeCl3��Һ�ƽ��壺Fe3����3H2O Fe(OH)3(����)��3H�� Fe(OH)3(����)��3H�� |
| C�������ᴿ�г�ȥMg2����Mg2����2OH��=Mg(OH)2�� |
| D����ҵ�Ͼ�����ͭ��������Ӧ��Cu��2e��=Cu2�� |