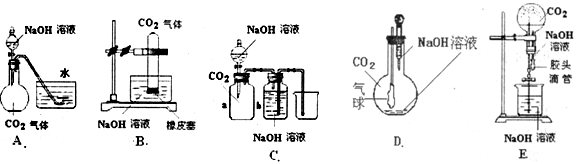
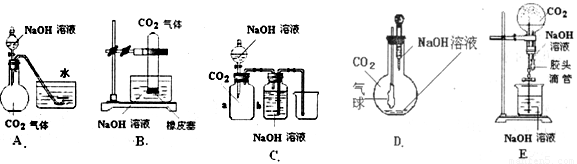
��Ŀ����
����״����һ�������CO2����ͨ�����ΪV L NaOH��Һ�У���ַ�Ӧ���ڼ�ѹ���µ�������������Һ���õ���ɫ���壮
��1������CO2ͨ������ͬ�����õ��İ�ɫ�������ɲ�ͬ���ƶϲ�д�����ֿ�����ɵĻ�ѧʽ�������Բ�������Ҳ����������ţ�
��______����______����______��
��______����______����______��
��2������Ӧ���Ⱥ�˳��д��������Ӧ�����ӷ���ʽ��______��
��3������Ӧ��CO2��NaOH����ʣ�࣬��Ӧ������Һ�м�������ij���ʯ��ˮ����m1g��ɫ������
�ٸ����������ݣ��ܷ������������״����CO2����������ܣ��ô���ʽ��ʾCO2�����V��CO2��=______�������ܣ�������______��
�ڸ����������ݣ��ܷ����������NaOH��Һ��Ũ�ȣ����ܣ��ô���ʽ��ʾNaOH��Һ��Ũ��c��NaOH��=______�������ܣ�������______��
�⣺��1���ٵ�CO2��NaOH�����ʵ�����С��1��2ʱ���ɷ�ӦCO2+2NaOH�TNa2CO3+H2O��֪��Ӧ����̼���ƣ�����ʣ���
NaOH����ѹ���µ�������������Һ���õ���ɫ����ΪNaOH��Na2CO3 ��
�ڵ�������̼��NaOH�����ʵ���Ϊ1��2ʱ����ӦCO2+2NaOH�TNa2CO3+H2Oǡ����ȫ���У�����Һ�е�����ΪNa2CO3����ѹ���µ�������������Һ���õ���ɫ����ΪNa2CO3 ��
�۵�������̼��NaOH�����ʵ����ȴ���1��2����С��1��1ʱ������CO2+2NaOH�TNa2CO3+H2O��CO2+NaOH�TNaHCO3����ѹ���µ�������������Һ���õ���ɫ����ΪNa2CO3��NaHCO3 ��
�ܵ�������̼��NaOH�����ʵ����ȡ�1��1ʱ������CO2+NaOH�TNaHCO3����ѹ���µ�������������Һ���õ���ɫ����Ϊ
NaHCO3��
�ʴ�Ϊ��NaOH��Na2CO3 ��Na2CO3��Na2CO3��NaHCO3 ��NaHCO3��
��2����CO2����ͨ��NaOH��Һ�У��ȷ���CO2+2NaOH�TNa2CO3+H2O������Na2CO3+H2O+CO2�TNaHCO3��
���ӷ�Ӧ�ֱ�ΪCO2+2OH-=CO32-+H2O��CO32-+H2O+CO2=2HCO3-��
�ʴ�Ϊ��CO2+2OH-=CO32-+H2O��CO32-+H2O+CO2=2HCO3-��
��3�������ɫ����Ϊ̼��ƣ���̼ԭ���غ��֪����״���¶�����̼�����Ϊ ��22.4L/mol=0.224m1L��
��22.4L/mol=0.224m1L��
�ʴ�Ϊ��0.224m1L��
�ڷ�Ӧ��CO2��NaOH����ʣ�࣬��Һ�е����ʿ���ΪNa2CO3��Ҳ����ΪNaHCO3��������ΪNa2CO3��NaHCO3 ����������ij���ʯ��ˮ����m1g��ɫ������ֻ����̼ԭ���غ�����̼�����ʵ���������ΪNa2CO3��NaHCO3 ��������֪����Ϣ�����������ӵ����ʵ��������ܼ����NaOH��Һ��Ũ�ȣ�
�ʴ�Ϊ�����ܣ���ΪNa2CO3��NaHCO3 ��������֪����Ϣ�����������ӵ����ʵ��������ܼ����NaOH��Һ��Ũ�ȣ�
��������1������CO2+2NaOH�TNa2CO3+H2O��CO2+NaOH�TNaHCO3��������
��2�����ݷ�Ӧ������̼���ƣ�������̼����������д���ӷ�Ӧ����ʽ��
��3���ٰ�ɫ����Ϊ̼��ƣ�����̼ԭ���غ�ɼ��������̼�������
����Ӧ������ʲ�ȷ����������NaOH��Һ��Ũ�ȣ�
���������⿼���йع�������ļ��㣬��ȷ�����Ļ�ѧ��Ӧ����ѧ�����ü��˼��衢ԭ���غ�������ǽ����Ĺؼ����ѶȲ���
NaOH����ѹ���µ�������������Һ���õ���ɫ����ΪNaOH��Na2CO3 ��
�ڵ�������̼��NaOH�����ʵ���Ϊ1��2ʱ����ӦCO2+2NaOH�TNa2CO3+H2Oǡ����ȫ���У�����Һ�е�����ΪNa2CO3����ѹ���µ�������������Һ���õ���ɫ����ΪNa2CO3 ��
�۵�������̼��NaOH�����ʵ����ȴ���1��2����С��1��1ʱ������CO2+2NaOH�TNa2CO3+H2O��CO2+NaOH�TNaHCO3����ѹ���µ�������������Һ���õ���ɫ����ΪNa2CO3��NaHCO3 ��
�ܵ�������̼��NaOH�����ʵ����ȡ�1��1ʱ������CO2+NaOH�TNaHCO3����ѹ���µ�������������Һ���õ���ɫ����Ϊ
NaHCO3��
�ʴ�Ϊ��NaOH��Na2CO3 ��Na2CO3��Na2CO3��NaHCO3 ��NaHCO3��
��2����CO2����ͨ��NaOH��Һ�У��ȷ���CO2+2NaOH�TNa2CO3+H2O������Na2CO3+H2O+CO2�TNaHCO3��
���ӷ�Ӧ�ֱ�ΪCO2+2OH-=CO32-+H2O��CO32-+H2O+CO2=2HCO3-��
�ʴ�Ϊ��CO2+2OH-=CO32-+H2O��CO32-+H2O+CO2=2HCO3-��
��3�������ɫ����Ϊ̼��ƣ���̼ԭ���غ��֪����״���¶�����̼�����Ϊ
 ��22.4L/mol=0.224m1L��
��22.4L/mol=0.224m1L���ʴ�Ϊ��0.224m1L��
�ڷ�Ӧ��CO2��NaOH����ʣ�࣬��Һ�е����ʿ���ΪNa2CO3��Ҳ����ΪNaHCO3��������ΪNa2CO3��NaHCO3 ����������ij���ʯ��ˮ����m1g��ɫ������ֻ����̼ԭ���غ�����̼�����ʵ���������ΪNa2CO3��NaHCO3 ��������֪����Ϣ�����������ӵ����ʵ��������ܼ����NaOH��Һ��Ũ�ȣ�
�ʴ�Ϊ�����ܣ���ΪNa2CO3��NaHCO3 ��������֪����Ϣ�����������ӵ����ʵ��������ܼ����NaOH��Һ��Ũ�ȣ�
��������1������CO2+2NaOH�TNa2CO3+H2O��CO2+NaOH�TNaHCO3��������
��2�����ݷ�Ӧ������̼���ƣ�������̼����������д���ӷ�Ӧ����ʽ��
��3���ٰ�ɫ����Ϊ̼��ƣ�����̼ԭ���غ�ɼ��������̼�������
����Ӧ������ʲ�ȷ����������NaOH��Һ��Ũ�ȣ�
���������⿼���йع�������ļ��㣬��ȷ�����Ļ�ѧ��Ӧ����ѧ�����ü��˼��衢ԭ���غ�������ǽ����Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ