جâؤ؟ؤعبف
¼×حé؟ةضئ³ة؛د³ةئّ(CO،¢H2)£¬شظضئ³ة¼×´¼£¬´ْجوبصزو¹©س¦½ôصإµؤب¼سح£®
زرضھ£؛¢ظCH4(g)£«H2O(g)£½CO(g)£«3H2(g)£»،÷H1£½£«206.2 kJ،¤mol£1
¢عCH4(g)£«![]() O2(g)£½CO(g)£«2H2(g)£»،÷H2£½£35.4 kJ،¤mol£1
O2(g)£½CO(g)£«2H2(g)£»،÷H2£½£35.4 kJ،¤mol£1
¢غCH4(g)£«2H2O(g)£½CO2(g)£«4H2(g)£»،÷H3£½£«165.0 kJ،¤mol£1
(1)CH4(g)سëCO2(g)·´س¦ةْ³ةCO(g)؛حH2(g)µؤبب»¯ر§·½³جت½خھ________£®
(2)´سشءدر،شٌ؛حؤـش´ہûسأ½ا¶ب£¬±ب½د·½·¨¢ظ؛ح¢ع£¬خھ؛د³ة¼×´¼£¬سأ¼×حéضئ؛د³ةئّµؤتتزث·½·¨خھ________(جîذٍ؛إ)£¬ئنشزٍتا________£®
(3)؛د³ةئّضذµؤH2؟ةسأسعةْ²ْNH3£¬شع½ّبë؛د³ةثا°³£سأCu(NH3)2Acبـز؛ہ´خüتصئنضذµؤCO£¬·ہض¹؛د³ةثضذµؤ´ك»¯¼ءضذ¶¾£¬ئن·´س¦تا£؛
Cu(NH3)2Ac£«CO£«NH3![]() [Cu(NH3)3]Ac،¤CO£»،÷H£¼0
[Cu(NH3)3]Ac،¤CO£»،÷H£¼0
Cu(NH3)2Acبـز؛خüتصCOµؤتتزثةْ²ْجُ¼س¦تا________£®
(4)½«CH4ةè¼ئ³ةب¼ءدµç³ط£¬ئنہûسأآت¸ü¸ك£¬×°ضأت¾زâبçدآح¼(A،¢Bخھ¶à؟×ذشج¼°ô)£®³ضذّح¨بë¼×ح飬شع±ê×¼×´؟ِدآ£¬دû؛ؤ¼×حéجه»V L£®
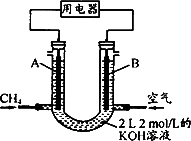
¢ظO£¼V،ـ44.8 Lت±£¬µç³ط×ـ·´س¦·½³جت½خھ________£»
¢ع44.8 L£¼V،ـ89.6 Lت±£¬¸؛¼«µç¼«·´س¦خھ________£»
¢غV£½67.2 Lت±£¬بـز؛ضذہë×سإ¨¶ب´َذ،¹طدµخھ________£®
½âخِ£؛
،،،،(1)CH4(g)£«CO2(g)£½2CO(g)£«2H2(g)£»،÷H£½£«247.4 kJ،¤mol£1(2·ض)
،،،،(2)¢ع(2·ض)£»ر،شٌCH4²»حêب«ب¼ةصضئ؛د³ةئّت±£¬·إ³ِببء؟£¬ح¬ت±µأµ½µؤCO،¢H2خïضتµؤء؟ض®±بخھ1،أ2£¬ؤـا،؛أحêب«·´س¦؛د³ة¼×´¼£®(2·ض)
،،،،(3)µحخآ،¢¸كر¹(2·ض)
،،،،(4)¢ظCH4£«2O2£«2KOH£½K2CO3£«3H2O(2·ض)
،،،،¢عCH![]() 8e££«9CO
8e££«9CO![]() £«3H2O£½10HCO
£«3H2O£½10HCO![]() (2·ض)
(2·ض)
،،،،¢غc(K+)£¾c(HCO![]() )£¾c(CO
)£¾c(CO![]() )£¾c(OH£)£¾c(H+)(2·ض)
)£¾c(OH£)£¾c(H+)(2·ض)

 ہّشإتéزµتî¼ظدخ½سؤ²¨³ِ°وةçدµءذ´ً°¸
ہّشإتéزµتî¼ظدخ½سؤ²¨³ِ°وةçدµءذ´ً°¸