��Ŀ����
����Ŀ�������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)����������֪��
��Na2S2O3��5H2O����ɫ������,������ˮ,��ϡ��Һ��BaCl2��Һ����������ɡ�
����Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3��
��BaSO3������ˮ,������ϡHCl��
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)
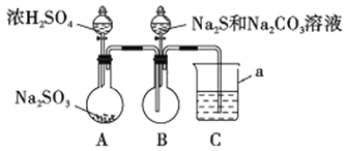
��1������a��������_________;C�е��Լ�������______ (ѡ��������ĸ���)��
A��ϡH2SO4 B������KMnO4��Һ C������NaHSO3��Һ D��NaOH��Һ
��2����ʵ��װ�������ȱ��,��Ľ�������_________________________��
��3��д��B�з�Ӧ�����ӷ���ʽ_________________________________________��
��4��A��B�з�Ӧ���,�ڲ�װ��ǰ��Ӧ��������Ⱦ�������ж������ȥ,���õķ����;��������________________________________________��
��5���÷����ò�Ʒ�г���������Na2SO3��Na2SO4��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4,��С�����������ʵ�鷽��,�뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ��������BaCl2��Һ,�а�ɫ�������ɣ�_______________��������δ��ȫ�ܽ�,���д̼�����ζ���������,���ȷ����Ʒ�к���Na2SO3��Na2SO4��
��6���ⶨ��Ʒ���ȣ�ȷ��ȡWg��Ʒ,����������ˮ�ܽ�,�Ե�����ָʾ��,��0.1000mol/L��ı���Һ�ζ���(��Ӧԭ��Ϊ��2S2O32-+I2=S4O62-+2I-)
�ٵζ����յ�ʱ,��Һ��ɫ�ı仯��_______________________��
�ڵζ���¼�������±���
�ζ�ǰ����/mL | �ζ������/mL | |
��һ�� | 0.10 | 16.12 |
�ڶ��� | 1.10 | 17.08 |
������ | 1.45 | 19.45 |
���Ĵ� | 0.00 | 16.00 |
�۲�Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
���𰸡� �ձ� BD ��AB��BC֮�����ӷ������İ�ȫƿ CO32-+2S2-+4SO2=3S2O32-+CO2 ����A����ƿ��������NaHCO3��Һ,�ٵμ�ϡH2SO4,ֱ��������CO2���彫װ���������ž�Ϊֹ ����,������ˮϴ�ӳ���,������м�������ϡHCl ����ɫ����ɫ�����ڰ�����ڲ���ɫ ![]() %
%
��������(1) ����a���������ձ���װ��A�в�����SO2��װ��B�еĻ����Һ��Ӧ����B�г����������г���CO2�⣬�����ܺ���H2S��SO2���ж�����Ⱦ���������壬����װ��C����β�����գ�H2S��SO2�����л�ԭ�ԣ�������������������KMnO4��Һ��Ӧ��ˮ��Һ���������ԣ�����Ӧ������װ��C�е��Լ�ΪB��D��
(2)ʵ������У�������һ���ȶ�������Ӧ��AB��BC֮�����ӷ������İ�ȫƿ��
(3) B�з�Ӧ�����ӷ���ʽCO32-+2S2-+4SO2=3S2O32-+CO2��
(4)Ϊ��ȥװ���ڵ��ж����壬���������彫װ���ڲ�����������װ��C�У����в�����������A����ƿ��������NaHCO3��Һ���ٵμ�ϡH2SO4��ֱ��������CO2���彫װ���������ž�Ϊֹ��
(5)���������˳�������������ˮϴ�ӣ�Ȼ��������м�������ϡHCl��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(6)�ٸ��ݷ�Ӧԭ��2S2O32-+I2=S4O62-+2I-���õ�����ָʾ�����յ�ʱ��Һ��ɫ�ı仯������ɫ����ɫ�����ڰ�����ڲ���ɫ���ڷ����Ĵ�ʵ�����ݿ�֪��������ʵ���������̫�����ţ���������������ʵ������������õ�ˮ����Һ�����Ϊ16.00mL����n(I2)=1.6��10-3mol�����Բ�Ʒ�Ĵ���=![]() ��100%=
��100%= ![]() %��
%��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJmol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJmol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJmol-1 | K3 |
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g) ��H
CH3OCH3(g)��CO2(g) ��H
�÷�Ӧ��H��__________________����ѧƽ�ⳣ��K��____________________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g) ![]() CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч���� D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����_______________________________��


