��Ŀ����
��֪�����Ȼ�֮����Ũ������������ȥһ����ˮ�����������磺

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;_______________��
(2)д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ____________________��
(3)д��F���ܵĽṹ��ʽ____________________��
(4)д��A�Ľṹ��ʽ________________________��
(5)1 mol C�ֱ�������Ľ���Na\,NaOH��Ӧ������Na��NaOH���ʵ���֮����_______��
(6)д��D��������(���廯�ƺ�Ũ����Ļ����)���ȷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
(1)ɱ������ (2)CH3COOH+H2O2��CH3COOOH+H2O

(5)4��3 (6)CH3CH2CH2CH2��OH+H��Br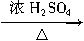 CH3CH2CH2CH2Br+H2O
CH3CH2CH2CH2Br+H2O
��������
�����������1�������������ǿ�����ԣ���ɱ��������
��2������B�ķ���ʽ��֪��BӦ�������ᣬ�����ԭ���غ��֪��EӦ����˫��ˮ�����Ը÷�Ӧ�Ļ�ѧ����ʽ��CH3COOH+H2O2 CH3COOOH+H2O��
CH3COOOH+H2O��
��3������C��F�Ľṹ��ʽ�Լ���Ӧ������֪��C����F�ķ�Ӧ�������ǻ�����ȥ��Ӧ������F�Ľṹ��ʽ������ ������Ϊ�����Ȼ�֮����Ũ������������ȥһ����ˮ��������������F�Ľṹ��ʽҲ������
������Ϊ�����Ȼ�֮����Ũ������������ȥһ����ˮ��������������F�Ľṹ��ʽҲ������ ��
�� ��
��
��4������G�Ľṹ�����ʿ�֪��G�Ƕ�ȩ����D��1������������A�Ľṹ��ʽ�� ��
��
��5��C�к���3���Ȼ���1���ǻ�������1 mol C�ֱ�������Ľ���Na\,NaOH��Ӧ������Na��NaOH���ʵ���֮����4:3��
��6��D��1���������������ᷢ���ǻ���ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽ��)CH3CH2CH2CH2��OH+H��Br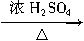 CH3CH2CH2CH2Br+H2O��
CH3CH2CH2CH2Br+H2O��
���㣺����ṹ��ʽ���л���ṹ�����ʡ�ͬ���칹����ж��Լ�����ʽ����д
�����������Ǹ߿��еij������ͣ������ۺ���ǿ���ѶȽϴ�ѧ����˼ά��������˸��ߵ�Ҫ������ע�ػ���֪ʶ������ѵ����ͬʱ �������������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�����ͳ���˼ά�������Լ�֪ʶ��Ǩ�����������ѧ���ķ������⡢������������������Ĺؼ����������պø����л������ɡ��ṹ�����ʡ��������ϵ�Լ���Ҫ�����ŵ���������ȥ�Ȼ���֪ʶ



 ��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��
��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��
 ��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D������ͼ����
��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D������ͼ���� 

