МвДҝДЪИЭ
ЈЁ2001ДкИ«№ъёЯҝјМвЈ©ТСЦӘFe2O3ФЪёЯВҜЦРУРПВБР·ҙУҰЈәFe2O3Ј«CO2ЎъFeOЈ« CO2Ј¬·ҙУҰРОіЙөД№ММе»мәПОпЈЁә¬Fe2O3әНFeOЈ©ЦРЈ¬ФӘЛШМъәНСхөДЦКБҝұИУГmЈЁFeЈ©ЎГ mЈЁOЈ©ұнКҫЎЈ
ЈЁ1Ј©ЙПКц№ММе»мәПОпЦРЈ¬mЈЁFeЈ©ЎГmЈЁOЈ©І»ҝЙДЬКЗ________ЈЁСЎМоaЎўbЎўcЈ¬¶аСЎ ҝЫ1·ЦЈ©ЎЈ
( )
A. 21ЎГ9 b. 21ЎГ7.5 c. 2lЎГ6
ЈЁ2Ј©ИфmЈЁFeЈ©ЎГmЈЁOЈ©ЈҪ21ЎГ8Ј¬јЖЛгFe2O3ұ»CO»№ФӯөД°Щ·ЦВКЎЈ
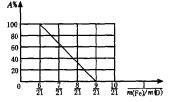
ЈЁ3Ј©ЙиFe2O3ұ»CO»№ФӯөД°Щ·ЦВКОӘAЈҘЈ¬ФтAЈҘәН»мәПОпЦРmЈЁFeЈ©ЎГmЈЁOЈ©өД№ШПөКҪОӘЎІУГә¬mЈЁFeЈ©ЎўmЈЁOЈ©өДҙъКэКҪ ұнКҫЎіAЈҘЈҪ________ЎЈ
ЗлФЪПВНјЦР»ӯіцAЈҘәНmЈЁFeЈ©ЎГmЈЁOЈ©№ШПөөДНјРОЎЈ
ЈЁ4Ј©Из№ыFe2O3әНCOөД·ҙУҰ·ЦБҪІҪҪшРРЈә
3Fe2O3Ј«COЎъ2Fe3O4Ј«CO2 Fe3O4Ј«COЎъ3FeOЈ«CO2КФ·ЦОц·ҙУҰРОіЙөД№ММе»мәПОпҝЙДЬөДЧйіЙј°ПаУҰөДmЈЁFeЈ©
ЎГmЈЁOЈ©ЎІБоmЈЁFeЈ©ЎГmЈЁOЈ©ЈҪ21ЎГaЈ¬РҙіцdөДИЎЦө·¶О§ЎіЎЈҪ«Ҫб№ыМоИлПВұнЎЈ
|
»мәПОпЧйіЙЈЁУГ»ҜС§КҪұнКҫЈ© |
aөДИЎЦө·¶О§ |
|
|
|
|
|
|
|
|
|
ҪвОцЈә
ЈЁ1Ј©ЈЁacЈ©
ЈЁ1Ј©ЎЎЎЎ ЙиFe2O3ФӯУРnomolЈ¬»№ФӯФӯ·ЦВКОӘAЈҘЈ¬
ФтУР![]() ЈҪ
ЈҪ![]() ЈҪ
ЈҪ![]() Ј¬A%ЈҪ
Ј¬A%ЈҪ![]() ЈҪ33.3%
ЈҪ33.3%
ЈЁ3Ј©3Јӯ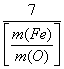 јыПВНј
јыПВНј
ЈЁ4Ј©Fe2O3ЎўFe3O4 8ЈјaЈј9 Fe2O3ЎўFeO 6ЈјaЈј8 Fe2O3ЎўFe3O4 FeO 6ЈјaЈј9
