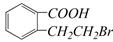��Ŀ����
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ����ͼ��ʾ:



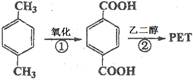
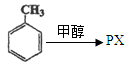
 ��֪R-CH=CHOH(ϩ��)���ȶ�,�ܿ�ת��Ϊ
��֪R-CH=CHOH(ϩ��)���ȶ�,�ܿ�ת��Ϊ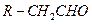 ��
��

 ����������Ϣ�ش���������:
����������Ϣ�ش���������:

 (1) A�ķ���ʽΪ ;
(1) A�ķ���ʽΪ ;

 (2) ��Ӧ�ڵĻ�ѧ����ʽ�� ;
(2) ��Ӧ�ڵĻ�ѧ����ʽ�� ;

 (3) A�Ľṹ��ʽ�� ;
(3) A�Ľṹ��ʽ�� ;

 (4) ��Ӧ�ٵĻ�ѧ����ʽ��
(4) ��Ӧ�ٵĻ�ѧ����ʽ��

 ;
;

 (5) A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� ��
(5) A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� ��

 ��
��

 ��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ

 ��
��




 ��֪R-CH=CHOH(ϩ��)���ȶ�,�ܿ�ת��Ϊ
��֪R-CH=CHOH(ϩ��)���ȶ�,�ܿ�ת��Ϊ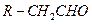 ��
��
 ����������Ϣ�ش���������:
����������Ϣ�ش���������:
 (1) A�ķ���ʽΪ ;
(1) A�ķ���ʽΪ ;
 (2) ��Ӧ�ڵĻ�ѧ����ʽ�� ;
(2) ��Ӧ�ڵĻ�ѧ����ʽ�� ;
 (3) A�Ľṹ��ʽ�� ;
(3) A�Ľṹ��ʽ�� ;
 (4) ��Ӧ�ٵĻ�ѧ����ʽ��
(4) ��Ӧ�ٵĻ�ѧ����ʽ�� 
 ;
;
 (5) A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� ��
(5) A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� ��
 ��
��
 ��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ 
 ��
��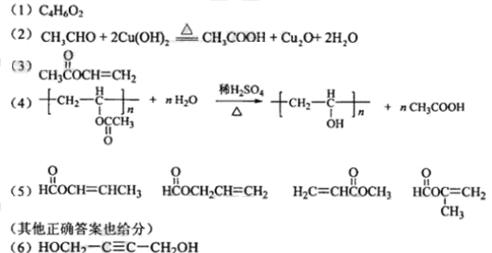
���⿼���л���Ľṹ�ƶϺ����ʣ�ע��ṹ��ʽ��ͬ���칹�塢��ѧ����ʽ����д���������⣬A������̼��ԭ����Ϊ4������ԭ�ӵĸ���Ϊ6��������ԭ�ӵĸ���Ϊ2������A�ķ���ʽΪC4H6O2������A���Է����ۺϷ�Ӧ��˵������̼̼˫����������������������������л����Ϊ����ˮ�⣬��A�к������������ݲ����Ͷȣ�û�����������š���A��ˮ�����C��D�Ĺ�ϵ���ж�C��D�е�̼ԭ������ȣ���Ϊ��������A�ĽṹΪCH3COOCH=CH2�����A������������ˮ���õ�CH3COOH��CH2=CHOH��̼̼˫����̼ԭ���Ͻ��ǻ����ȶ���ת��ΪCH3CHO��A�ۺϷ�Ӧ��õ��IJ���BΪ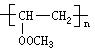 ��������������ˮ�⣬�������������
��������������ˮ�⣬�������������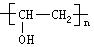 ��
��
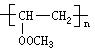 ��������������ˮ�⣬�������������
��������������ˮ�⣬�������������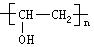 ��
��
��ϰ��ϵ�д�
�����Ŀ

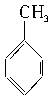
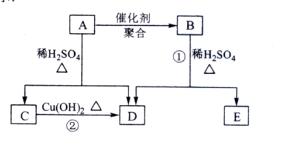
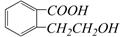 ��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�
��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�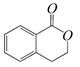 C��
C��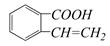
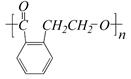 E��
E��