��Ŀ����
��1��49gH2SO4�����ʵ����� ���������Ƴ�200mL��Һ��������Һ�����ʵ���Ũ��Ϊ �����к�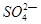 ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��2��������ء���������������ɵĻ����Һ����PH=1��c(Al3+)=0.4mol/L, c(![]() )=0.8mol/ L, ��c(K+)Ϊ ��
)=0.8mol/ L, ��c(K+)Ϊ ��
��3������״����a L HCl(g)����1000 gˮ�У��õ�������ܶ�Ϊbg/cm3�������������ʵ���Ũ���� ��
��1�� 0.5 �� 2.5mol/L�� 3.01��1023 �� 22.4L ��2��0.3 mol/L��2�֣���
��3��![]() ��2�֣���
��2�֣���
����:

��ϰ��ϵ�д�
�����Ŀ
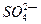 ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��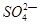 ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��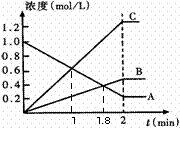 ��2����ͼ��ʾ800��ʱ��A��B��C������������
��2����ͼ��ʾ800��ʱ��A��B��C������������