��Ŀ����
��10�֣�������Mg������100mL 1.5mol��L��1ϡH2SO4�У���Ӧ��������ȥ����Mg�ۣ���Һ��t��������������Һ����Ϊ72.0gʱ��ʼ����MgSO4��xH2O���壬����������12.3gʱ��ʣ����Һ48.0g��ͨ��������ش��������⣨��д��������̣���
��1�����ɱ�״���µ����������
��2����ʼ����MgSO4��xH2O����ʱ��Һ������������
��3��MgSO4��xH2O�е�xֵ��
��1��3.36L ��2��25.0%��3��7
����:n��H2SO4��=0.1L��1.5mol��L��1=0.15mol
��1��Mg �� H2SO4 = MgSO4 �� H2��
0.15mol 0.15mol 0.15mol
V��H2��=22.4L��mol��1��0.15mol=3.36L
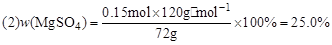
��3��

��ϰ��ϵ�д�
�����Ŀ


