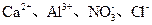��Ŀ����
������ԭ�ζ�ԭ��ͬ�к͵ζ�ԭ�����ƣ�Ϊ�˲ⶨijδ֪Ũ�ȵ�NaHSO3��Һ��Ũ�ȣ�����0.1000mol/L������KMnO4��Һ���еζ����ش��������⣺
��1����ƽ���ӷ���ʽ�� MnO4-+ HSO3-+ H+���� Mn2++ SO42-+ H2O
��2���ζ������У�NaHSO3��Һ�������20.00mL������0.100mol/L������KMnO4��Һ16.00mL����NaHSO3��Һ�����ʵ���Ũ���� mol/L��
��3�����в����ᵼ�²ⶨ���ƫ�ߵ���
E���۲����ʱ���ζ�ǰ���ӣ��ζ�����

��1����ƽ���ӷ���ʽ�� MnO4-+ HSO3-+ H+���� Mn2++ SO42-+ H2O
��2���ζ������У�NaHSO3��Һ�������20.00mL������0.100mol/L������KMnO4��Һ16.00mL����NaHSO3��Һ�����ʵ���Ũ���� mol/L��
��3�����в����ᵼ�²ⶨ���ƫ�ߵ���
| A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ��� |
| B���ζ�ǰ��ƿδ���� |
| C���ζ�ǰ�ζ��ܼ��첿�������� |
| D����С�Ľ���������KMnO4��Һ������ƿ�� |
��1��2��5��1��2��5��3 ��2��0.2000����3��ACD
��1�������غ������ƽ��2MnO4-+5HSO3-+H+==2Mn2++5SO42-+3H2O��
��2��n(NaHSO3)=2.5n(KMnO4)=2.5��0.1��0.016mol=0.004mol, C(NaHSO3)= 0.2000 mol/L;
(3) ACD
��2��n(NaHSO3)=2.5n(KMnO4)=2.5��0.1��0.016mol=0.004mol, C(NaHSO3)= 0.2000 mol/L;
(3) ACD

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 A��0.1 mol?L-1 pHΪ4��NaHB��Һ�У�c(HB-)��c(H2B)��c(B2-)
A��0.1 mol?L-1 pHΪ4��NaHB��Һ�У�c(HB-)��c(H2B)��c(B2-)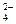 ��NO
��NO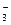
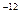 mol��L����Һ�У�HCO
mol��L����Һ�У�HCO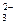 ��Na+��CO
��Na+��CO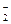
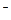 ��AlO
��AlO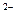
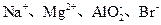 B
B 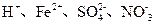
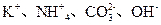 D
D