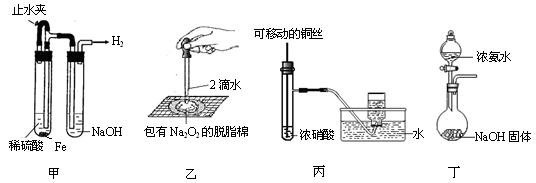
ÌâÄżÄÚÈĘ
žùŸĘÒȘÇóÍêłÉÏÂÁĐžśĐĄÌâ
Łš1Ł©ąÙÊ”ŃéÊÒÓĂŒÓÈÈčÌÌć»ìșÏÎï”Ä·œ·šÖƱž°±Æű”Ä»ŻŃ§·ŽÓŠ·œłÌÊœÊÇ ĄŁ
ąÚÎȘÁ˔ÔœžÉÔï”ÄNH3ŁŹÓĂ________ŚöžÉÔïŒÁĄŁŁšÌî±àșĆŁ©
Łš2Ł©H2OČÎÓë”ÄÖĂ»»·ŽÓŠŁș
·ûșÏX+WĄúY+VŁŹÒŃÖȘXșÍY·Ö±đÊǶÌÖÜÆÚÍŹÖśŚćÁœÖÖÔȘËŰĐγɔĔ„ÖÊŁŹ WĄąVÊÇ»ŻșÏÎï
ąÙWÊÇËźÇÒV”ÄŃæÉ«·ŽÓŠÎȘ»ÆÉ«ŁŹÀëŚÓ·œłÌÊœ Ł»
ąÚVÊÇËźŁŹ»ŻŃ§·œłÌÊœÎȘ ĄŁ
Łš1Ł©ąÙÊ”ŃéÊÒÓĂŒÓÈÈčÌÌć»ìșÏÎï”Ä·œ·šÖƱž°±Æű”Ä»ŻŃ§·ŽÓŠ·œłÌÊœÊÇ ĄŁ
ąÚÎȘÁ˔ÔœžÉÔï”ÄNH3ŁŹÓĂ________ŚöžÉÔïŒÁĄŁŁšÌî±àșĆŁ©
| AŁźŒîÊŻ»Ò | BŁźĆšH2SO4 | CŁźÎȚËźCaCl2 | DŁźP2O5 |
·ûșÏX+WĄúY+VŁŹÒŃÖȘXșÍY·Ö±đÊǶÌÖÜÆÚÍŹÖśŚćÁœÖÖÔȘËŰĐγɔĔ„ÖÊŁŹ WĄąVÊÇ»ŻșÏÎï
ąÙWÊÇËźÇÒV”ÄŃæÉ«·ŽÓŠÎȘ»ÆÉ«ŁŹÀëŚÓ·œłÌÊœ Ł»
ąÚVÊÇËźŁŹ»ŻŃ§·œłÌÊœÎȘ ĄŁ
(7·Ö)
Łš1Ł©ąÙ2NH4Cl + Ca(OH)2 2NH3Ąü + CaCl2 + 2H2OŁš2·ÖŁ©
2NH3Ąü + CaCl2 + 2H2OŁš2·ÖŁ©
ąÚAŁš1·ÖŁ©
(2)ąÙ2Na +2 H2O ="2Na+" +2OH- + H2ĄüŁš2·ÖŁ©
ąÚ2H2S+O2 ==2S+2H2OŁš2·ÖŁ©
Łš1Ł©ąÙ2NH4Cl + Ca(OH)2
 2NH3Ąü + CaCl2 + 2H2OŁš2·ÖŁ©
2NH3Ąü + CaCl2 + 2H2OŁš2·ÖŁ©ąÚAŁš1·ÖŁ©
(2)ąÙ2Na +2 H2O ="2Na+" +2OH- + H2ĄüŁš2·ÖŁ©
ąÚ2H2S+O2 ==2S+2H2OŁš2·ÖŁ©
ÊÔÌâ·ÖÎöŁșŁš1Ł©ąÙÊ”ŃéÊÒÓĂŒÓÈÈčÌÌć»ìșÏÎï”Ä·œ·šÖƱž°±Æű”Ä»ŻŃ§·ŽÓŠ·œłÌÊœÊÇ2NH4Cl + Ca(OH)2
 2NH3Ąü + CaCl2 + 2H2OąÚÎȘÁ˔ÔœžÉÔï”ÄNH3ŁŹÓĂAĄąŒîÊŻ»ÒŚöžÉÔïŒÁĄŁŁš2Ł©ąÙWÊÇËźÇÒV”ÄŃæÉ«·ŽÓŠÎȘ»ÆÉ«ŁŹË”ĂśVÖĐșŹÓĐÄÆÔȘËŰŁŹÀëŚÓ·œłÌÊœ2Na +2 H2O ="2Na+" +2OH- + H2ĄüąÚVÊÇËźŁŹ »ŻŃ§·œłÌÊœÎȘ2H2S+O2 ==2S+2H2OĄŁ
2NH3Ąü + CaCl2 + 2H2OąÚÎȘÁ˔ÔœžÉÔï”ÄNH3ŁŹÓĂAĄąŒîÊŻ»ÒŚöžÉÔïŒÁĄŁŁš2Ł©ąÙWÊÇËźÇÒV”ÄŃæÉ«·ŽÓŠÎȘ»ÆÉ«ŁŹË”ĂśVÖĐșŹÓĐÄÆÔȘËŰŁŹÀëŚÓ·œłÌÊœ2Na +2 H2O ="2Na+" +2OH- + H2ĄüąÚVÊÇËźŁŹ »ŻŃ§·œłÌÊœÎȘ2H2S+O2 ==2S+2H2OĄŁ
Á·Ï°ČáÏ”ÁĐŽđ°ž
 ÔĶÁżìł”Ï”ÁĐŽđ°ž
ÔĶÁżìł”Ï”ÁĐŽđ°ž
ÏàčŰÌâÄż
 ÒÒ
ÒÒ ±ûĄŁÏÂÁĐÓĐčŰÎïÖÊ”ÄÍƶÏČ»ŐęÈ·”ÄÊÇ (ĄĄĄĄ)ĄŁ
±ûĄŁÏÂÁĐÓĐčŰÎïÖÊ”ÄÍƶÏČ»ŐęÈ·”ÄÊÇ (ĄĄĄĄ)ĄŁ