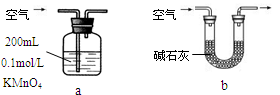
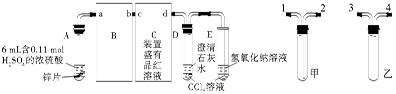��Ŀ����
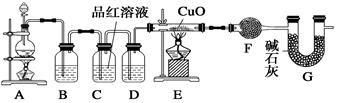
ij�о���ѧϰС��ͨ������ʵ��̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3���������Ʊ�����ͭ���塣���������ա���ͬѧ��װ��I����ʵ�飬���ȷ�Ӧ�������ڣ�����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᡣ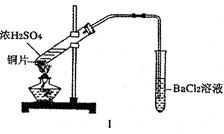
��1����ɫ������ ��
��2����ͬѧ�Ͱ�ɫ����������ԭ����������ּ��裬�����ּ�������ǣ�
�� ��
��ͬѧ����˸Ľ�װ�â����ʵ�飬�����ͬѧ����ļ��裨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��
�ٴ��ɼУ�ͨ��N2һ��ʱ���رյ��ɼУ�
�ڵμ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�
��3�������ٵ�Ŀ���� ������ƿB�е��Լ��� ��
��4����ʵ���ܷ�����ͬѧ�������ּ����е�����һ�� �������� ��
��ͬѧ��Ӧ�����ɫ��Һ�м���������CuO�����˺���Һ�Ƴ�����ͭ���壨CuSO4��xH2O�������ü��ȷ��ⶨ�þ����нᾧˮx��ֵ��ʵ�����ݼ�¼���£�
| �������� | �����뾧�������� | ���Ⱥ���������������� | |
| ��һ�γ��� | �ڶ��γ��� | ||
| 11.710g | 22.700g | 18.621g | a |
��5���������ٽ��е����γ�������a����ֵ��ΧӦΪ ��
��6�������Ⱥ����������������Ϊ18.620g������x ��ʵ��ֵ ��������λС������������ ��
��1��BaSO4��2�֣���
��2����Ũ����������������Ȼ�����Һ���ɳ�����1�֣�
��SO2�������������������ɳ�����1�֣�
��3���ų�װ���ڵĿ�����O2����1�֣� ����NaHSO3��Һ��1�֣�
��4�����ܣ�1�֣�����Ϊͬʱ�ı���������������ȷ���������ĸ����������á���1�֣�
��5��18.621��0.001 ��1�֣�
��6��5.25 ��2�֣� +5%��1�֣�
���������������1����ɫ�������������ᣬ��˰�ɫ���������ᱵ��
��2����������ķе�ͼ����¶ȷ�����˵��Ũ�������¶Ƚϸ�ʱҲ�ܻӷ���H2SO4����������BaSO3�ܹ��������ᣬ����Ԫ���غ��֪�ó���ֻ����BaSO4���п���һ�ǻӷ�����H2SO4�ṩ��SO42-��Ba2+��Ӧ���ɵģ�Ҳ�п�������Һ���ܽ��������SO2���ò��������ᣬ�����õ�BaSO4��
��3�����������ȶ����ž�װ���еĿ����е�����������֤����ԭ���Ƿ���ȷ��Ϊ��ȥ��Ӧ�д�������״���ᣬ���Խ�����ͨ������NaHSO3��Һ��
��4������ͬʱ�ı���������������ȷ���������ĸ����������á�
��5����������ʵ�����ܳ���0.001�������������ٽ��е����γ�������a����ֵ��ΧӦΪ18.621��0.001��
��6�������Ⱥ����������������Ϊ18.620g�����ʧȥ�Ľᾧˮ����Ϊ22.700g ��18.620g��4.08g��ˮ�����ʵ�����0.227mol�����������Ϊ22.700g��11.710g��10.99g���� ��x��0.227
��x��0.227
���x��5.25�����������Ϊ ��100%��5%��
��100%��5%��
���㣺����ʵ�鷽�������̽��������ͭ����ᾧˮ�����ļ���

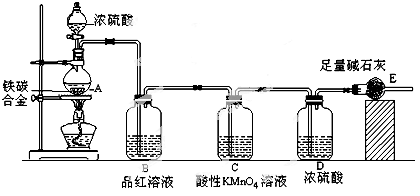
��18�֣�ʵ���ҳ�������װ���Ʊ����ռ������������壬��̽�������ʡ�
��1��װ��A�еķ�Һ©����ʢװ��Һ��ͨ���� ��Բ����ƿ��Ԥ�ȼ������ͭƬ�������ļ۸��ͭ�ļ۸�ͣ��˴���ͭƬ������Ƭ��ԭ���� ��
��2�������D��װ�ĸ��������ˮ�Ȼ��ƣ��������� ��
��3������ʱ������ƣ�Ũ������ϡ��װ��E������������β�������չ����з�����Ӧ�Ļ�ѧ����ʽ���£�2NO2��2NaOH��NaNO3��NaNO2��H2O ��NO2��NO��2NaOH��2NaNO2��H2O
��NO��NO2����������ɿɱ�ʾ��NOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵ����Ϊ ������ĸ��
| A��1.1 | B��1.2 | C��1.5 | D��1.8 |
��4��������װ���У������������ռ�װ�ã�����ռ�������������NO2���壬����õ��ռ�װ���� (ѡ�F���� ��G��)��
����(H2S)��һ�־��г�������ζ����ɫ���壬�о綾�������ڶ������������Լ���Ȼ���С�������ĺܶ�����������Ҳ������Ҫ���á�
���ϣ���H2S������ˮ?Լ1��2?����ˮ��ҺΪ��Ԫ���ᡣ
��H2S��������������ӷ�Ӧ���ɳ�����
��H2S�ڿ�����ȼ�գ�����ʵ���ɫ��
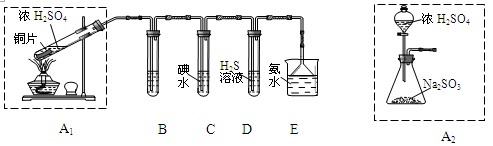
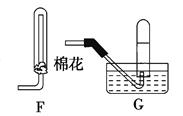
(1)ij��ѧС���������ȡH2S����֤�����ʵ�ʵ�飬����ͼ��ʾ��A����CuSO4��Һ��B�з���ʪ�����ɫʯ����ֽ��C����FeCl3��Һ��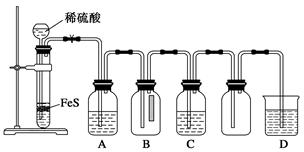
�ش��������⣺
��A���к�ɫ����(CuS)������A�з�����Ӧ�Ļ�ѧ����ʽΪ___________________��
��B�������__________________��
��C��ֻ��dz��ɫ��������������Һ��dz��ɫ����C�з�����Ӧ�����ӷ���ʽΪ______________��
��D��ʢ�ŵ��Լ�������________(����ĸ���)��
a��ˮ b������
c��NaCl��Һ d��NaOH��Һ
(2)Ϊ��һ��̽����2����Ļ������룫4����Ļ����ﷴӦ������С��ͬѧ�����������ʵ�顣
| | ʵ����� | ʵ������ |
| ʵ��1 | ����Ũ�ȵ�Na2S��Na2SO3��Һ�������2��1��� | ���������� |
| ʵ��2 | ��H2Sͨ��Na2SO3��Һ�� | δ�����Գ������ټ�������ϡ���ᣬ������������dz��ɫ���� |
| ʵ��3 | ��SO2ͨ��Na2S��Һ�� | ��dz��ɫ�������� |
��֪������ƽ�ⳣ����
H2S��Kal��1.3��10��7��Ka2��7.1��10��15
H2SO3��Ka1��1.7��10��2��Ka2��5.6��10��8
�ٸ�������ʵ�飬���Եó����ۣ���__________�����£���4���������������2����Ļ����
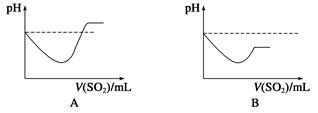
�ڽ�SO2����ͨ��H2Sˮ��Һ��ֱ�����������б�ʾ��ҺpH��SO2��������仯��ϵʾ��ͼ��ȷ����________(����ĸ���)��

(3)�����أ�������H2S����Ag�����û���Ӧ����H2���ֽ�H2S����ͨ��װ�����۵IJ����ܣ�����Ƽ�ʵ�飬ͨ�����鷴Ӧ����֤��H2S��Ag�������û���Ӧ______��
ijУ��ѧ��ȤС��Ϊ̽������Ũ���ᷴӦ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣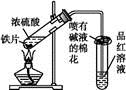
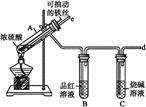
��������ͼ1������������������ͼ2
(1)�Ƚ���ʵ��װ��,ͼ2װ�õ��ŵ���:
���ܸ��õ������ж�����SO2,��ֹ����Ⱦ����;
������(2)������e��������Ҫ����:һ���ڷ�Ӧ������,�ܲ���Һ����,����Һ�⡱������ֹSO2�����ݳ�����Ⱦ����;��������(3)��˵����SO2���������ʵ������������(4)��Ӧһ��ʱ���,�õι���ȡA�Թ��е���Һ��������ˮ��Ϊ����,�����������������ӵijɷ����������ֿ���:
��:ֻ����Fe3+;��:ֻ����Fe2+;��:����Fe3+����Fe2+��
Ϊ��֤��Ŀ�����,ѡ�������Լ�,��д���пո�:
| A��ϡHCl��Һ | B��ϡH2SO4��Һ |
| C��KSCN��Һ | D��KMnO4��Һ |
G.H2O2��Һ
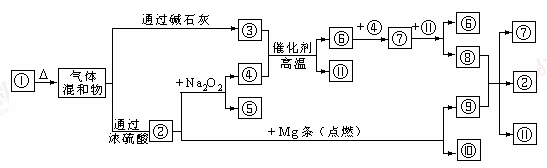
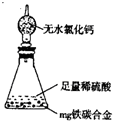
 ZnSO4��SO2����2H2O��ȡ22.4 L(��״��)SO2���塣ȡ65.0 gп����98%��ŨH2SO4(�ѣ�1.84 g��cm-3)110 mL��ַ�Ӧ��пȫ���ܽ⡣�����Ƶõ����壬��ͬѧ��Ϊ���ܻ���������Ϊ�ˣ���ѧС���ͬѧ���������ʵ��װ�ã�������ȡ���������̽����
ZnSO4��SO2����2H2O��ȡ22.4 L(��״��)SO2���塣ȡ65.0 gп����98%��ŨH2SO4(�ѣ�1.84 g��cm-3)110 mL��ַ�Ӧ��пȫ���ܽ⡣�����Ƶõ����壬��ͬѧ��Ϊ���ܻ���������Ϊ�ˣ���ѧС���ͬѧ���������ʵ��װ�ã�������ȡ���������̽����