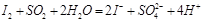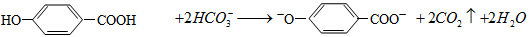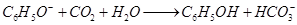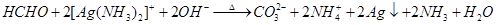��Ŀ����
�������ӷ���ʽ��������ʵ�������ȷ����
A����FeCl3��Һ��ȡFe(OH)3���壺Fe3+ + 3H2O  Fe(OH)3���� + 3H+[�� Fe(OH)3���� + 3H+[�� |
| B��С�մ�������������Һ��ϣ�HCO3�� + OH�� = CO2��+H2O |
| C����������Һ�м������������������Һ��Al3+ + SO42�� + Ba2+ + 4OH�� = BaSO4�� + AlO2�� + 2H2O |
| D����ˮ������ͨ����CO2��SiO32�� + CO2 + H2O = H2SiO3�� + HCO3�� |
A
B����������̼���ƺ�ˮ��C��������������ʱ������ʽΪKAl(SO4)2��2Ba(OH)2=2BaSO4��KAlO2��2H2O����C�Ǵ���ġ�D�е�ɲ��غ㣬��ȷ����SiO32�� + 2CO2 + H2O = H2SiO3�� + 2HCO3����������ȷ�Ĵ���A��

��ϰ��ϵ�д�
�����Ŀ
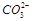 +H2O
+H2O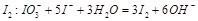
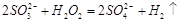
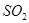 ������ɫ��Һ��
������ɫ��Һ��